Trafficking dynamics of glycosylated pannexin 1 proteins
- PMID: 18649184
- PMCID: PMC2528835
- DOI: 10.1080/15419060802013885
Trafficking dynamics of glycosylated pannexin 1 proteins
Abstract
Pannexins are mammalian orthologs of innexins and have a predicted topological folding pattern similar to that of connexins, except they are glycosylated. Rat pannexin 1 is glycosylated at N254 and this residue is important for plasma membrane targeting. Here we demonstrate that cell surface expression levels of the rat pannexin 1 N254Q mutant are rescued by coexpression with the wild-type protein. In paired Xenopus oocytes, the functional effect of this rescue is inconsequential; however, cell surface deglycosylation by PNGase F significantly enhanced functional gap junction formation. In mammalian cells, wild-type oligomers traffic at a slower rate than Myc-or tetracysteine domain-tagged versions, a behavior opposite to that of tagged connexins. The temporal differences of Panx1 trafficking correlate with spatial differences of intracellular localizations induced by Golgi blockage by Brefeldin-A or glycosylation prevention by tunicamycin. Therefore, Panx1 has kinetics and dynamics that make it unique to serve distinct functions separate from connexin-based channels.
Figures
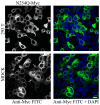
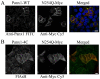



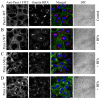

References
-
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. - PubMed
-
- Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem. 2007;282:31733–31743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
