Evaluation of E1A double mutant oncolytic adenovectors in anti-glioma gene therapy
- PMID: 18649343
- PMCID: PMC2365750
- DOI: 10.1002/jmv.21264
Evaluation of E1A double mutant oncolytic adenovectors in anti-glioma gene therapy
Abstract
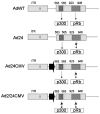
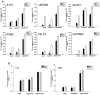
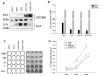
Malignant glioma, in particular glioblastoma multiforme (GBM), represents one of the most devastating cancers currently known and existing treatment regimens do little to change patient prognosis. Conditionally replicating adenoviral vectors (CRAds) represent attractive experimental anti-cancer agents with potential for clinical application. However, early protein products of the wild type adenovirus backbone--such as E1A--limit CRAds' replicative specificity. In this study, we evaluated the oncolytic potency and specificity of CRAds in which p300/CPB and/or pRb binding capacities of E1A were ablated to reduce non-specific replicative cytolysis. In vitro cytopathic assays, quantitative PCR analysis, Western blot, and flow cytometry studies demonstrate the superior anti-glioma efficacy of a double-mutated CRAd, Ad2/24CMV, which harbors mutations that reduce E1A binding to p300/CPB and pRb. When compared to its single-mutated and wild type counterparts, Ad2/24CMV demonstrated attenuated replication and cytotoxicity in representative normal human brain while displaying enhanced replicative cytotoxicity in malignant glioma. These results have implications for the development of double-mutated CRAd vectors for enhanced GBM therapy.
Figures

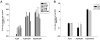
Similar articles
-
Pharmacokinetic study of neural stem cell-based cell carrier for oncolytic virotherapy: targeted delivery of the therapeutic payload in an orthotopic brain tumor model.Cancer Gene Ther. 2012 Jun;19(6):431-42. doi: 10.1038/cgt.2012.21. Epub 2012 May 4. Cancer Gene Ther. 2012. PMID: 22555507 Free PMC article.
-
Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo.Gene Ther. 2009 Feb;16(2):262-78. doi: 10.1038/gt.2008.165. Epub 2008 Dec 11. Gene Ther. 2009. PMID: 19078993 Free PMC article.
-
Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma.Hum Gene Ther. 2007 Jul;18(7):589-602. doi: 10.1089/hum.2007.002. Hum Gene Ther. 2007. PMID: 17630837
-
Engineering regulatory elements for conditionally-replicative adeno-viruses.Curr Gene Ther. 2003 Aug;3(4):357-85. doi: 10.2174/1566523034578311. Curr Gene Ther. 2003. PMID: 12871022 Review.
-
Concepts in Oncolytic Adenovirus Therapy.Int J Mol Sci. 2021 Sep 29;22(19):10522. doi: 10.3390/ijms221910522. Int J Mol Sci. 2021. PMID: 34638863 Free PMC article. Review.
Cited by
-
Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma.Mol Ther. 2011 Sep;19(9):1714-26. doi: 10.1038/mt.2011.100. Epub 2011 May 31. Mol Ther. 2011. PMID: 21629227 Free PMC article.
-
Oncolytic virotherapy for malignant glioma: translating laboratory insights into clinical practice.Front Oncol. 2013 Feb 25;3:32. doi: 10.3389/fonc.2013.00032. eCollection 2013. Front Oncol. 2013. PMID: 23443138 Free PMC article.
-
Gene therapy for brain cancer: combination therapies provide enhanced efficacy and safety.Curr Gene Ther. 2009 Oct;9(5):409-21. doi: 10.2174/156652309789753301. Curr Gene Ther. 2009. PMID: 19860655 Free PMC article. Review.
-
Novel therapies in glioblastoma.Neurol Res Int. 2012;2012:428565. doi: 10.1155/2012/428565. Epub 2012 Feb 28. Neurol Res Int. 2012. PMID: 22530121 Free PMC article.
-
Oncolytic adenoviruses: A thorny path to glioma cure.Genes Dis. 2014 Dec;1(2):214-226. doi: 10.1016/j.gendis.2014.09.009. Genes Dis. 2014. PMID: 25685829 Free PMC article.
References
-
- Baluchamy S, Sankar N, Navaraj A, Moran E, Thimmapaya B. Relationship between E1A binding to cellular proteins, c-myc activation and S-phase induction. Oncogene. 2007;26:781–787. - PubMed
-
- Bandara LR, La Thangue NB. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991;351:494–497. - PubMed
-
- Banerjee NS, Rivera AA, Wang M, Chow LT, Broker TR, Curiel DT, Nettelbeck DM. Analyses of melanoma-targeted oncolytic adenoviruses with tyrosinase enhancer/promoter-driven E1A, E4, or both in submerged cells and organotypic cultures. Mol Cancer Ther. 2004;3:437–449. - PubMed
-
- Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

