Recovery from monocular deprivation using binocular deprivation
- PMID: 18650311
- PMCID: PMC2576197
- DOI: 10.1152/jn.90411.2008
Recovery from monocular deprivation using binocular deprivation
Abstract
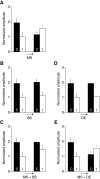
Ocular dominance (OD) plasticity is a robust paradigm for examining the functional consequences of synaptic plasticity. Previous experimental and theoretical results have shown that OD plasticity can be accounted for by known synaptic plasticity mechanisms, using the assumption that deprivation by lid suture eliminates spatial structure in the deprived channel. Here we show that in the mouse, recovery from monocular lid suture can be obtained by subsequent binocular lid suture but not by dark rearing. This poses a significant challenge to previous theoretical results. We therefore performed simulations with a natural input environment appropriate for mouse visual cortex. In contrast to previous work, we assume that lid suture causes degradation but not elimination of spatial structure, whereas dark rearing produces elimination of spatial structure. We present experimental evidence that supports this assumption, measuring responses through sutured lids in the mouse. The change in assumptions about the input environment is sufficient to account for new experimental observations, while still accounting for previous experimental results.
Figures


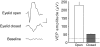

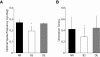
References
-
- Blais BS, Intrator N, Shouval HZ, Cooper LN. Receptive field formation in natural scene environments. Comparison of single-cell learning rules. Neural Comput 10: 1797–1813, 1998. - PubMed
-
- Drager UC, Olsen JF. Origins of crossed and uncrossed retinal projections in pigmented and albino mice. J Comp Neurol 191: 383–412, 1980. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

