Ca2+-dependent, phospholipid-binding residues of synaptotagmin are critical for excitation-secretion coupling in vivo
- PMID: 18650324
- PMCID: PMC2949296
- DOI: 10.1523/JNEUROSCI.0197-08.2008
Ca2+-dependent, phospholipid-binding residues of synaptotagmin are critical for excitation-secretion coupling in vivo
Abstract
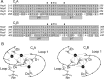
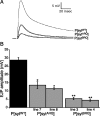
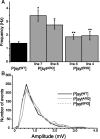
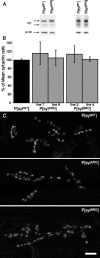
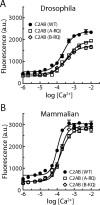
Synaptotagmin I is the Ca(2+) sensor for fast, synchronous release of neurotransmitter; however, the molecular interactions that couple Ca(2+) binding to membrane fusion remain unclear. The structure of synaptotagmin is dominated by two C(2) domains that interact with negatively charged membranes after binding Ca(2+). In vitro work has implicated a conserved basic residue at the tip of loop 3 of the Ca(2+)-binding pocket in both C(2) domains in coordinating this electrostatic interaction with anionic membranes. Although results from cultured cells suggest that the basic residue of the C(2)A domain is functionally significant, such studies provide contradictory results regarding the importance of the C(2)B basic residue during vesicle fusion. To directly test the functional significance of each of these residues at an intact synapse in vivo, we neutralized either the C(2)A or the C(2)B basic residue and assessed synaptic transmission at the Drosophila neuromuscular junction. The conserved basic residues at the tip of the Ca(2+)-binding pocket of both the C(2)A and C(2)B domains mediate Ca(2+)-dependent interactions with anionic membranes and are required for efficient evoked transmitter release. Our results directly support the hypothesis that the interactions between synaptotagmin and the presynaptic membrane, which are mediated by the basic residues at the tip of both the C(2)A and C(2)B Ca(2+)-binding pockets, are critical for coupling Ca(2+) influx with vesicle fusion during synaptic transmission in vivo. Our model for synaptotagmin's direct role in coupling Ca(2+) binding to vesicle fusion incorporates this finding with results from multiple in vitro and in vivo studies.
Figures


Similar articles
-
Membrane penetration by synaptotagmin is required for coupling calcium binding to vesicle fusion in vivo.J Neurosci. 2011 Feb 9;31(6):2248-57. doi: 10.1523/JNEUROSCI.3153-09.2011. J Neurosci. 2011. PMID: 21307261 Free PMC article.
-
Calcium binding by synaptotagmin's C2A domain is an essential element of the electrostatic switch that triggers synchronous synaptic transmission.J Neurosci. 2012 Jan 25;32(4):1253-60. doi: 10.1523/JNEUROSCI.4652-11.2012. J Neurosci. 2012. PMID: 22279210 Free PMC article.
-
The C2A domain of synaptotagmin is an essential component of the calcium sensor for synaptic transmission.PLoS One. 2020 Feb 7;15(2):e0228348. doi: 10.1371/journal.pone.0228348. eCollection 2020. PLoS One. 2020. PMID: 32032373 Free PMC article.
-
Synaptotagmin: Mechanisms of an electrostatic switch.Neurosci Lett. 2020 Mar 23;722:134834. doi: 10.1016/j.neulet.2020.134834. Epub 2020 Feb 10. Neurosci Lett. 2020. PMID: 32057923 Review.
-
Roles of SNARE proteins and synaptotagmin I in synaptic transmission: studies at the Drosophila neuromuscular synapse.Neurosignals. 2003 Jan-Feb;12(1):13-30. doi: 10.1159/000068912. Neurosignals. 2003. PMID: 12624525 Review.
Cited by
-
Different states of synaptotagmin regulate evoked versus spontaneous release.Nat Commun. 2016 Mar 22;7:10971. doi: 10.1038/ncomms10971. Nat Commun. 2016. PMID: 27001899 Free PMC article.
-
Membrane penetration by synaptotagmin is required for coupling calcium binding to vesicle fusion in vivo.J Neurosci. 2011 Feb 9;31(6):2248-57. doi: 10.1523/JNEUROSCI.3153-09.2011. J Neurosci. 2011. PMID: 21307261 Free PMC article.
-
Calcium binding by synaptotagmin's C2A domain is an essential element of the electrostatic switch that triggers synchronous synaptic transmission.J Neurosci. 2012 Jan 25;32(4):1253-60. doi: 10.1523/JNEUROSCI.4652-11.2012. J Neurosci. 2012. PMID: 22279210 Free PMC article.
-
Protein determinants of SNARE-mediated lipid mixing.Biophys J. 2010 Jul 21;99(2):553-60. doi: 10.1016/j.bpj.2010.04.060. Biophys J. 2010. PMID: 20643074 Free PMC article.
-
Synaptotagmin oligomerization is essential for calcium control of regulated exocytosis.Proc Natl Acad Sci U S A. 2018 Aug 7;115(32):E7624-E7631. doi: 10.1073/pnas.1808792115. Epub 2018 Jul 23. Proc Natl Acad Sci U S A. 2018. PMID: 30038018 Free PMC article.
References
-
- Araç D, Chen X, Khant HA, Ubach J, Ludtke SJ, Kikkawa M, Johnson AE, Chiu W, Südhof TC, Rizo J. Close membrane-membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat Struct Mol Biol. 2006;13:209–217. - PubMed
-
- Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat Struct Mol Biol. 2004;11:36–44. - PubMed
-
- Banker G, Goslin K, editors. Culturing nerve cells. Ed 2. Cambridge, MA: MIT; 1998.
-
- Bekkers JM, Stevens CF. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990;346:724–729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
