Learning-specific changes in long-term depression in adult perirhinal cortex
- PMID: 18650332
- PMCID: PMC6670846
- DOI: 10.1523/JNEUROSCI.1935-08.2008
Learning-specific changes in long-term depression in adult perirhinal cortex
Abstract
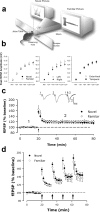
Learning is widely believed to involve synaptic plasticity, using mechanisms such as those used in long-term potentiation (LTP). We assess whether the mechanisms used in alternative forms of plasticity, long-term depression (LTD) and depotentiation, play a role in learning. We have exploited the involvement of the perirhinal cortex in two different forms of learning to compare simultaneously, within the same brain region, their effects on LTD and depotentiation. Multiple-exposure learning but not single-exposure learning in vivo prevented, in a muscarinic receptor-dependent manner, subsequent induction of LTD and depotentiation, but not LTP, in perirhinal cortex in vitro. The contrast in the effects of the two types of learning under these particular experimental conditions indicate that the in vitro change is unlikely to be attributable to synapse-specific plastic changes registering the precise details of the individual learned associations. Instead, it is concluded that the lack of LTD and depotentiation arises from, and establishes the importance of, a learning-related generalized change in plasticity gain. The existence of this additional mechanism has important implications for interpretations of how plasticity relates to learning.
Figures



Similar articles
-
Nitric oxide-dependent long-term depression but not endocannabinoid-mediated long-term potentiation is crucial for visual recognition memory.J Physiol. 2013 Aug 15;591(16):3963-79. doi: 10.1113/jphysiol.2013.254862. Epub 2013 May 13. J Physiol. 2013. PMID: 23671159 Free PMC article.
-
L-type voltage-dependent calcium channel antagonists impair perirhinal long-term recognition memory and plasticity processes.J Neurosci. 2009 Jul 29;29(30):9534-44. doi: 10.1523/JNEUROSCI.5199-08.2009. J Neurosci. 2009. PMID: 19641116 Free PMC article.
-
Expression of long-term depression underlies visual recognition memory.Neuron. 2008 Apr 24;58(2):186-94. doi: 10.1016/j.neuron.2008.02.022. Neuron. 2008. PMID: 18439404
-
Mechanisms of synaptic plasticity and recognition memory in the perirhinal cortex.Prog Mol Biol Transl Sci. 2014;122:193-209. doi: 10.1016/B978-0-12-420170-5.00007-6. Prog Mol Biol Transl Sci. 2014. PMID: 24484702 Review.
-
NMDA receptor-dependent long-term depression in the anterior cingulate cortex.Rev Neurosci. 2006;17(4):403-13. doi: 10.1515/revneuro.2006.17.4.403. Rev Neurosci. 2006. PMID: 17139841 Review.
Cited by
-
Dual functions of perirhinal cortex in fear conditioning.Hippocampus. 2012 Oct;22(10):2068-79. doi: 10.1002/hipo.22058. Epub 2012 Aug 18. Hippocampus. 2012. PMID: 22903623 Free PMC article. Review.
-
Mechanisms of memory storage in a model perirhinal network.Brain Struct Funct. 2017 Jan;222(1):183-200. doi: 10.1007/s00429-016-1210-4. Epub 2016 Mar 12. Brain Struct Funct. 2017. PMID: 26971254 Free PMC article.
-
Characterization of auditory synaptic inputs to gerbil perirhinal cortex.Front Neural Circuits. 2015 Aug 14;9:40. doi: 10.3389/fncir.2015.00040. eCollection 2015. Front Neural Circuits. 2015. PMID: 26321918 Free PMC article.
-
Depletion of perineuronal nets enhances recognition memory and long-term depression in the perirhinal cortex.J Neurosci. 2013 Apr 17;33(16):7057-65. doi: 10.1523/JNEUROSCI.6267-11.2013. J Neurosci. 2013. PMID: 23595763 Free PMC article.
-
Visual recognition memory: a view from V1.Curr Opin Neurobiol. 2015 Dec;35:57-65. doi: 10.1016/j.conb.2015.06.008. Epub 2015 Jul 4. Curr Opin Neurobiol. 2015. PMID: 26151761 Free PMC article. Review.
References
-
- Abraham WC, Robins A. Memory retention—the synaptic stability versus plasticity dilemma. Trends Neurosci. 2005;28:73–78. - PubMed
-
- Aigner TG, Mishkin M. Scopolamine impairs recall of one-trial stimulus reward association in monkeys. Behav Brain Res. 1993;54:133–136. - PubMed
-
- Anderson WW, Collingridge GL. The LTP Program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Methods. 2001;108:71–83. - PubMed
-
- Bliss TVP, Collingridge GL. A synaptic model of memory-long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
