Lessons from the first comprehensive molecular characterization of cell cycle control in rodent insulinoma cell lines
- PMID: 18650366
- PMCID: PMC2570402
- DOI: 10.2337/db08-0393
Lessons from the first comprehensive molecular characterization of cell cycle control in rodent insulinoma cell lines
Abstract
Objective: Rodent insulinoma cell lines may serve as a model for designing continuously replicating human beta-cell lines and provide clues as to the central cell cycle regulatory molecules in the beta-cell.
Research design and methods: We performed a comprehensive G1/S proteome analysis on the four most widely studied rodent insulinoma cell lines and defined their flow cytometric profiles and growth characteristics.
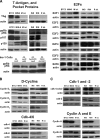
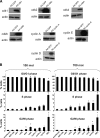
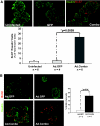
Results: 1) Despite their common T-antigen-derived origins, MIN6 and BTC3 cells display markedly different G1/S expression profiles; 2) despite their common radiation origins, RINm5F and INS1 cells display striking differences in cell cycle protein profiles; 3) phosphorylation of pRb is absent in INS1 and RINm5F cells; 4) cyclin D2 is absent in RINm5F and BTC3 cells and therefore apparently dispensable for their proliferation; 5) every cell cycle inhibitor is upregulated, presumably in a futile attempt to halt proliferation; 6) among the G1/S proteome members, seven are pro-proliferation molecules: cyclin-dependent kinase-1, -2, -4, and -6 and cyclins A, E, and D3; and 7) overexpression of the combination of these seven converts arrested proliferation rates in primary rat beta-cells to those in insulinoma cells. Unfortunately, this therapeutic overexpression appears to mildly attenuate beta-cell differentiation and function.
Conclusions: These studies underscore the importance of characterizing the cell cycle at the protein level in rodent insulinoma cell lines. They also emphasize the hazards of interpreting data from rodent insulinoma cell lines as modeling normal cell cycle progression. Most importantly, they provide seven candidate targets for inducing proliferation in human beta-cells.
Figures



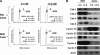

References
-
- Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocana A, Vasavada RC, Stewart AF: Molecular control of cell cycle progression in the pancreatic beta cell. Endocrine Reviews 27: 356–370, 2006 - PubMed
-
- Heit JJ, Karnik SK, Kim SK: Intrinsic regulators of pancreatic beta-cell proliferation. Annu Rev Cell Dev Biol 22: 311–338, 2006 - PubMed
-
- Hohmeier H, Newgard CB: Cell lines derived from pancreatic islets. Mol Cell Endocrinol 228: 121–128, 2004 - PubMed
-
- Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB: Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130: 167–178, 1992 - PubMed

