Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses
- PMID: 18650384
- PMCID: PMC2492502
- DOI: 10.1073/pnas.0802423105
Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses
Erratum in
- Proc Natl Acad Sci U S A. 2008 Sep 23;105(38):14744
Abstract
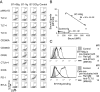
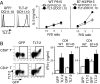
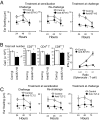
The B7 family member B7-H3 (CD276) plays important roles in immune responses. However, the function of B7-H3 remains controversial. We found that murine B7-H3 specifically bound to Triggering receptor expressed on myeloid cells (TREM)-like transcript 2 (TLT-2, TREML2). TLT-2 was expressed on CD8(+) T cells constitutively and on activated CD4(+) T cells. Stimulation with B7-H3 transfectants preferentially up-regulated the proliferation and IFN-gamma production of CD8(+) T cells. Transduction of TLT-2 into T cells resulted in enhanced IL-2 and IFN-gamma production via interactions with B7-H3. Blockade of the B7-H3:TLT-2 pathway with a mAb against B7-H3 or TLT-2 efficiently inhibited contact hypersensitivity responses. Our results demonstrate a direct interaction between B7-H3 and TLT-2 that preferentially enhances CD8(+) T cell activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
The contrasting role of B7-H3.Proc Natl Acad Sci U S A. 2008 Jul 29;105(30):10277-8. doi: 10.1073/pnas.0805458105. Epub 2008 Jul 23. Proc Natl Acad Sci U S A. 2008. PMID: 18650376 Free PMC article. No abstract available.
References
-
- Carreno BM, Collins M. The B7 family of ligands and its receptors: New pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. - PubMed
-
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. - PubMed
-
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. - PubMed
-
- Chapoval AI, et al. B7–H3: A costimulatory molecule for T cell activation and IFN-γ production. Nat Immunol. 2001;2:269–274. - PubMed
-
- Ferlazzo G, et al. T lymphocytes express B7 family molecules following interaction with dendritic cells and acquire bystander costimulatory properties. Eur J Immunol. 2002;32:3092–3101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

