A novel mechanism by which thiazolidinediones facilitate the proteasomal degradation of cyclin D1 in cancer cells
- PMID: 18650423
- PMCID: PMC2546561
- DOI: 10.1074/jbc.M802160200
A novel mechanism by which thiazolidinediones facilitate the proteasomal degradation of cyclin D1 in cancer cells
Abstract
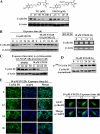
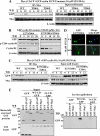
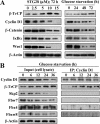
This study identifies a novel mechanism by which thiazolidinediones mediate cyclin D1 repression in prostate cancer cells. Based on the finding that the thiazolidinedione family of peroxisome proliferator-activated receptor gamma (PPARgamma) agonists mediated PPARgamma-independent cyclin D1 degradation, we developed a novel PPARgamma-inactive troglitazone derivative, STG28, with high potency in cyclin D1 ablation. STG28-mediated cyclin D1 degradation was preceded by Thr-286 phosphorylation and nuclear export, which however, were independent of glycogen synthase kinase 3beta. Mutational analysis further confirmed the pivotal role of Thr-286 phosphorylation in STG28-induced nuclear export and proteolysis. Of several kinases examined, inhibition of IkappaB kinase alpha blocked STG28-mediated cytoplasmic sequestration and degradation of cyclin D1. Pulldown of ectopically expressed Cul1, the scaffold protein of the Skp-Cullin-F-box E3 ligase, in STG28-treated cells revealed an increased association of cyclin D1 with beta-TrCP, whereas no specific binding was noted with other F-box proteins examined, including Skp2, Fbw7, Fbx4, and Fbxw8. This finding represents the first evidence that cyclin D1 is targeted by beta-TrCP. Moreover, beta-TrCP expression was up-regulated in response to STG28, and ectopic expression and small interfering RNA-mediated knock-down of beta-TrCP enhanced and protected against STG28-facilitated cyclin D1 degradation, respectively. Because cyclin D1 lacks the DSG destruction motif, mutational and modeling analyses indicate that cyclin D1 was targeted by beta-TrCP through an unconventional recognition site, (279)EEVDLACpT(286), reminiscent to that of Wee1. Moreover, we obtained evidence that this beta-TrCP-dependent degradation takes part in controlling cyclin D1 turnover when cancer cells undergo glucose starvation, which endows physiological relevance to this novel mechanism.
Figures






Similar articles
-
Thiazolidinediones modulate the expression of beta-catenin and other cell-cycle regulatory proteins by targeting the F-box proteins of Skp1-Cul1-F-box protein E3 ubiquitin ligase independently of peroxisome proliferator-activated receptor gamma.Mol Pharmacol. 2007 Sep;72(3):725-33. doi: 10.1124/mol.107.035287. Epub 2007 Jun 14. Mol Pharmacol. 2007. PMID: 17569795
-
Thiazolidinediones mimic glucose starvation in facilitating Sp1 degradation through the up-regulation of beta-transducin repeat-containing protein.Mol Pharmacol. 2009 Jul;76(1):47-57. doi: 10.1124/mol.109.055376. Epub 2009 Apr 16. Mol Pharmacol. 2009. PMID: 19372209 Free PMC article.
-
Regulation of GATA-binding protein 2 levels via ubiquitin-dependent degradation by Fbw7: involvement of cyclin B-cyclin-dependent kinase 1-mediated phosphorylation of THR176 in GATA-binding protein 2.J Biol Chem. 2015 Apr 17;290(16):10368-81. doi: 10.1074/jbc.M114.613018. Epub 2015 Feb 10. J Biol Chem. 2015. PMID: 25670854 Free PMC article.
-
Role of SKP1-CUL1-F-box-protein (SCF) E3 ubiquitin ligases in skin cancer.J Genet Genomics. 2013 Mar 20;40(3):97-106. doi: 10.1016/j.jgg.2013.02.001. Epub 2013 Feb 10. J Genet Genomics. 2013. PMID: 23522382 Free PMC article. Review.
-
SCF(Fbx4/alphaB-crystallin) E3 ligase: when one is not enough.Cell Cycle. 2008 Oct;7(19):2983-6. doi: 10.4161/cc.7.19.6775. Epub 2008 Oct 12. Cell Cycle. 2008. PMID: 18818515 Review.
Cited by
-
Recent advances in SCF ubiquitin ligase complex: Clinical implications.Biochim Biophys Acta. 2016 Aug;1866(1):12-22. doi: 10.1016/j.bbcan.2016.05.001. Epub 2016 May 5. Biochim Biophys Acta. 2016. PMID: 27156687 Free PMC article. Review.
-
The PPAR Gamma Agonist Troglitazone Regulates Erk 1/2 Phosphorylation via a PPARγ-Independent, MEK-Dependent Pathway in Human Prostate Cancer Cells.PPAR Res. 2012;2012:929052. doi: 10.1155/2012/929052. Epub 2012 Feb 20. PPAR Res. 2012. PMID: 22448169 Free PMC article.
-
FBXO43 increases CCND1 stability to promote hepatocellular carcinoma cell proliferation and migration.Front Oncol. 2023 Mar 3;13:1138348. doi: 10.3389/fonc.2023.1138348. eCollection 2023. Front Oncol. 2023. PMID: 36937431 Free PMC article.
-
Effects of an Indolocarbazole-Derived CDK4 Inhibitor on Breast Cancer Cells.J Cancer. 2011 Jan 8;2:36-51. doi: 10.7150/jca.2.36. J Cancer. 2011. PMID: 21234300 Free PMC article.
-
Recent advances with cyclin-dependent kinase inhibitors: therapeutic agents for breast cancer and their role in immuno-oncology.Expert Rev Anticancer Ther. 2019 Jul;19(7):569-587. doi: 10.1080/14737140.2019.1615889. Epub 2019 Jun 20. Expert Rev Anticancer Ther. 2019. PMID: 31219365 Free PMC article. Review.
References
-
- Diehl, J. A. (2002) Cancer Biol. Ther. 1226 -231 - PubMed
-
- Weinstein, I. B. (2002) Science 29763 -64 - PubMed
-
- Stacey, D. W. (2003) Curr. Opin. Cell Biol. 15158 -163 - PubMed
-
- Chung, D. C. (2004) Ann. N. Y. Acad. Sci. 1014209 -217 - PubMed
-
- Fu, M., Wang, C., Li, Z., Sakamaki, T., and Pestell, R. G. (2004) Endocrinology 1455439 -5447 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

