Activation of Polo-like kinase 3 by hypoxic stresses
- PMID: 18650425
- PMCID: PMC2533803
- DOI: 10.1074/jbc.M801326200
Activation of Polo-like kinase 3 by hypoxic stresses
Abstract
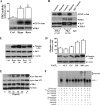
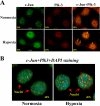
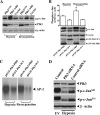
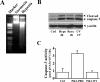
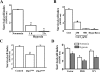
Hypoxia/reoxygenation stress induces the activation of specific signaling proteins and activator protein 1 (AP-1) to regulate cell cycle regression and apoptosis. In the present study, we report that hypoxia/reoxygenation stress activates AP-1 by increasing c-Jun phosphorylation and DNA binding activity through activation of Polo-like-kinase 3 (Plk3) resulting in apoptosis. The specific effect of hypoxia/reoxygenation stress on Plk3 activation resulting in c-Jun phosphorylation was the opposite of UV irradiation-induced responses that are meanly independent on activation of the stress-induced JNK signaling pathway in human corneal epithelial (HCE) cells. The effect of hypoxia/reoxygenation stress-induced Plk3 activation on increased c-Jun phosphorylation and apoptosis was also mimicked by exposure of cells to CoCl(2). Hypoxia/reoxygenation activated Plk3 in HCE cells to directly phosphorylate c-Jun proteins at phosphorylation sites Ser-63 and Ser-73, and to increase DNA binding activity of c-Jun, detected by EMSA. Further evidence demonstrated that Plk3 and phospho-c-Jun were immunocolocalized in the nuclear compartment of hypoxia/reoxygenation stress-induced cells. Increased Plk3 activity by overexpression of wild-type and dominantly positive Plk3 enhanced the effect of hypoxia/reoxygenation on c-Jun phosphorylation and cell death. In contrast, knocking-down Plk3 mRNA suppressed hypoxia-induced c-Jun phosphorylation. Our results provide a new mechanism indicating that hypoxia/reoxygenation induces Plk3 activation instead of the JNK effect to directly phosphorylate and activate c-Jun, subsequently contributing to apoptosis in HCE cells.
Figures
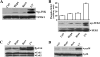
References
-
- Singh, N., Amin, S., Richter, E., Rashid, S., Scoglietti, V., Jani, P. D., Wang, J., Kaur, R., Ambati, J., Dong, Z., and Ambati, B. K. (2005) Investig. Ophthalmol. Vis. Sci. 46 1647–1652 - PubMed
-
- Singh, N., Jani, P. D., Suthar, T., Amin, S., and Ambati, B. K. (2006) Investig. Ophthalmol. Vis. Sci. 47 4787–4793 - PubMed
-
- Cursiefen, C., Maruyama, K., Jackson, D. G., Streilein, J. W., and Kruse, F. E. (2006) Cornea 25 443–447 - PubMed
-
- Mwaikambo, B. R., Sennlaub, F., Ong, H., Chemtob, S., and Hardy, P. (2006) Invest Ophthalmol. Vis. Sci. 47 4356–4364 - PubMed
-
- Zhang, M. C., Wang, Y., and Yang, Y. (2006) Ocul. Immunol. Inflamm. 14 359–365 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

