Amyloid precursor protein trafficking, processing, and function
- PMID: 18650430
- PMCID: PMC2573065
- DOI: 10.1074/jbc.R800019200
Amyloid precursor protein trafficking, processing, and function
Abstract
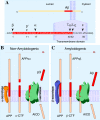
Intracellular trafficking and proteolytic processing of amyloid precursor protein (APP) have been the focus of numerous investigations over the past two decades. APP is the precursor to the amyloid beta-protein (Abeta), the 38-43-amino acid residue peptide that is at the heart of the amyloid cascade hypothesis of Alzheimer disease (AD). Tremendous progress has been made since the initial identification of Abeta as the principal component of brain senile plaques of individuals with AD. Specifically, molecular characterization of the secretases involved in Abeta production has facilitated cell biological investigations on APP processing and advanced efforts to model AD pathogenesis in animal models. This minireview summarizes salient features of APP trafficking and amyloidogenic processing and discusses the putative biological functions of APP.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

