Erythroblastic islands: niches for erythropoiesis
- PMID: 18650462
- PMCID: PMC2481536
- DOI: 10.1182/blood-2008-03-077883
Erythroblastic islands: niches for erythropoiesis
Abstract
Erythroblastic islands, the specialized niches in which erythroid precursors proliferate, differentiate, and enucleate, were first described 50 years ago by analysis of transmission electron micrographs of bone marrow. These hematopoietic subcompartments are composed of erythroblasts surrounding a central macrophage. A hiatus of several decades followed, during which the importance of erythroblastic islands remained unrecognized as erythroid progenitors were shown to possess an autonomous differentiation program with a capacity to complete terminal differentiation in vitro in the presence of erythropoietin but without macrophages. However, as the extent of proliferation, differentiation, and enucleation efficiency documented in vivo could not be recapitulated in vitro, a resurgence of interest in erythroid niches has emerged. We now have an increased molecular understanding of processes operating within erythroid niches, including cell-cell and cell-extracellular matrix adhesion, positive and negative regulatory feedback, and central macrophage function. These features of erythroblast islands represent important contributors to normal erythroid development, as well as altered erythropoiesis found in such diverse diseases as anemia of inflammation and chronic disease, myelodysplasia, thalassemia, and malarial anemia. Coupling of historical, current, and future insights will be essential to understand the tightly regulated production of red cells both in steady state and stress erythropoiesis.
Figures

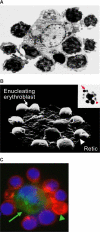
 ) and a multilobulated reticulocyte (
) and a multilobulated reticulocyte ( ). (C) Confocal immunofluorescence image of an island reconstituted from freshly harvested mouse bone marrow cells stained with erythroid-specific marker (red), macrophage marker (green) and DNA probe (blue). Central macrophage is indicated by an arrow and a multilobulated reticulocyte by an arrowhead. (Panels A and B are courtesy of Michel Prenant of France, and panel C is reprinted from Lee et al with permission from the American Society of Hematology.) Illustration by Paulette Dennis.
). (C) Confocal immunofluorescence image of an island reconstituted from freshly harvested mouse bone marrow cells stained with erythroid-specific marker (red), macrophage marker (green) and DNA probe (blue). Central macrophage is indicated by an arrow and a multilobulated reticulocyte by an arrowhead. (Panels A and B are courtesy of Michel Prenant of France, and panel C is reprinted from Lee et al with permission from the American Society of Hematology.) Illustration by Paulette Dennis.
References
-
- Bessis M. L'ilot erythroblastique. Unite functionelle de la moelle osseuse. Rev Hematol. 1958;13:8–11. - PubMed
-
- Bessis MC, Breton-Gorius J. Iron metabolism in the bone marrow as seen by electron microscopy: a critical review. Blood. 1962;19:635–663. - PubMed
-
- Mohandas N, Prenant M. Three-dimensional model of bone marrow. Blood. 1978;51:633–643. - PubMed
-
- Allen TD, Dexter TM. Ultrastructural aspects of erythropoietic differentiation in long-term bone marrow culture. Differentiation. 1982;21:86–94. - PubMed
-
- Bessis M, Mize C, Prenant M. Erythropoiesis: comparison of in vivo and in vitro amplification. Blood Cells. 1978;4:155–174. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

