Inhibition of SNAT2 by metabolic acidosis enhances proteolysis in skeletal muscle
- PMID: 18650482
- PMCID: PMC2573006
- DOI: 10.1681/ASN.2007101108
Inhibition of SNAT2 by metabolic acidosis enhances proteolysis in skeletal muscle
Abstract
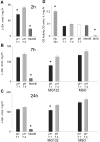
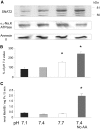
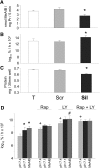
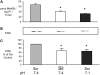
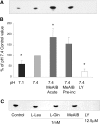
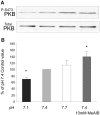
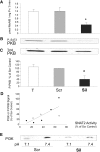
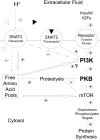
Insulin resistance is a major cause of muscle wasting in patients with ESRD. Uremic metabolic acidosis impairs insulin signaling, which normally suppresses proteolysis. The low pH may inhibit the SNAT2 l-Glutamine (L-Gln) transporter, which controls protein synthesis via amino acid-dependent insulin signaling through mammalian target of rapamycin (mTOR). Whether SNAT2 also regulates signaling to pathways that control proteolysis is unknown. In this study, inhibition of SNAT2 with the selective competitive substrate methylaminoisobutyrate or metabolic acidosis (pH 7.1) depleted intracellular L-Gln and stimulated proteolysis in cultured L6 myotubes. At pH 7.1, inhibition of the proteasome led to greater depletion of L-Gln, indicating that amino acids liberated by proteolysis sustain L-Gln levels when SNAT2 is inhibited by acidosis. Acidosis shifted the dose-response curve for suppression of proteolysis by insulin to the right, confirming that acid increases proteolysis by inducing insulin resistance. Blocking mTOR or phosphatidylinositol-3-kinase (PI3K) increased proteolysis, indicating that both signaling pathways are involved in its regulation. When both mTOR and PI3K were inhibited, methylaminoisobutyrate or acidosis did not stimulate proteolysis further. Moreover, partial silencing of SNAT2 expression in myotubes and myoblasts with small interfering RNA stimulated proteolysis and impaired insulin signaling through PI3K. In conclusion, SNAT2 not only regulates mTOR but also regulates proteolysis through PI3K and provides a link among acidosis, insulin resistance, and protein wasting in skeletal muscle cells.
Figures



References
-
- Siew ED, Pupim LB, Majchrzak KM, Shintani A, Flakoll PJ, Ikizler TA: Insulin resistance is associated with skeletal muscle protein breakdown in non-diabetic chronic hemodialysis patients. Kidney Int 71: 146–152, 2007 - PubMed
-
- Lee SW, Park GH, Lee SW, Song JH, Hong KC, Kim MJ: Insulin resistance and muscle wasting in non-diabetic end-stage renal disease patients. Nephrol Dial Transplant 22: 2554–2562, 2007 - PubMed
-
- Mitch WE: Metabolic and clinical consequences of metabolic acidosis. J Nephrol 19[Suppl 9]: S70–S75, 2006 - PubMed
-
- Evans K, Nasim Z, Brown J, Butler H, Kauser S, Varoqui H, Erickson JD, Herbert TP, Bevington A: Acidosis-sensing glutamine pump SNAT2 determines amino acid levels and mammalian target of rapamycin signalling to protein synthesis in L6 muscle cells. J Am Soc Nephrol 18: 1426–1436, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

