Paxillin comes of age
- PMID: 18650496
- PMCID: PMC2522309
- DOI: 10.1242/jcs.018044
Paxillin comes of age
Abstract
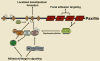
Paxillin is a multi-domain scaffold protein that localizes to the intracellular surface of sites of cell adhesion to the extracellular matrix. Through the interactions of its multiple protein-binding modules, many of which are regulated by phosphorylation, paxillin serves as a platform for the recruitment of numerous regulatory and structural proteins that together control the dynamic changes in cell adhesion, cytoskeletal reorganization and gene expression that are necessary for cell migration and survival. In particular, paxillin plays a central role in coordinating the spatial and temporal action of the Rho family of small GTPases, which regulate the actin cytoskeleton, by recruiting an array of GTPase activator, suppressor and effector proteins to cell adhesions. When paxillin was first described 18 years ago, the amazing complexity of cell-adhesion organization, dynamics and signaling was yet to be realized. Herein we highlight our current understanding of how the multiple protein interactions of paxillin contribute to the coordination of cell-adhesion function.
Figures


References
-
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971;67:359–67. - PubMed
-
- Ando R, Mizuno H, Miyawaki A. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science. 2004;306:1370–3. - PubMed
-
- Arold ST, Hoellerer MK, Noble ME. The structural basis of localization and signaling by the focal adhesion targeting domain. Structure (Camb) 2002;10:319–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

