Differential immunological phenotypes are exhibited after scald and flame burns
- PMID: 18650781
- PMCID: PMC2674561
- DOI: 10.1097/SHK.0b013e31817fbf4d
Differential immunological phenotypes are exhibited after scald and flame burns
Abstract
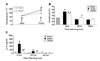
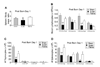
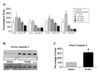
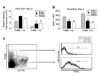
A dysfunctional immune system is known to be part of the pathophysiology after burn trauma. However, reports that support this have used a variety of methods, with numerous variables, to induce thermal injury. We hypothesized that, all other parameters being equal, an injury infliction by a scald would yield different immunological responses than one inflicted by a flame. Here, we demonstrated that both burn methods produced a full-thickness burn, yet there was more of an increase in subdermal temperature, hematocrit, mortality, and serum IL-6 concentrations associated with the scald burn. On postinjury day 1, the scald-burned mice showed diminished lymphocyte numbers, interferon gamma production, and lymphocyte T-bet expression as compared with sham- and flame-burned mice. On postburn day 8, spleens from both sets of thermally injured animals showed an increase in proinflammatory myeloid cells as compared with sham-burned mice. Furthermore, the T-cell numbers, T-bet expression, and phenotype were changed such that interferon gamma production was higher in scald-burned mice than in sham- and flame-burned mice. Altogether, the data show that differential immunological phenotypes were observed depending on the thermal injury method used.
Figures




References
-
- Stieritz DD, Holder IA. Experimental studies of the pathogenesis of Pseudomonas aeruginosa infection: evidence for the in-vivo production of a lethal toxin. J Med Microbiol. 1978;11:101–109. - PubMed
-
- Cribbs RK, Luquette MH, Besner GE. A standardized model of partial thickness scald burns in mice. J Surg Res. 1998;80:69–74. - PubMed
-
- Greenhalgh DG. Models of wound healing. J Burn Care Rehabil. 2005;26:293–305. - PubMed
-
- Patenaude J, D'Elia M, Hamelin C, Garrel D, Bernier J. Burn injury induces a change in T cell homeostasis affecting preferentially CD4+ T cells. J Leukoc Biol. 2005;77:141–150. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

