Modes of resistance to anti-angiogenic therapy
- PMID: 18650835
- PMCID: PMC2874834
- DOI: 10.1038/nrc2442
Modes of resistance to anti-angiogenic therapy
Abstract
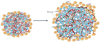
Angiogenesis inhibitors targeting the vascular endothelial growth factor (VEGF) signalling pathways are affording demonstrable therapeutic efficacy in mouse models of cancer and in an increasing number of human cancers. However, in both preclinical and clinical settings, the benefits are at best transitory and are followed by a restoration of tumour growth and progression. Emerging data support a proposition that two modes of unconventional resistance underlie such results: evasive resistance, an adaptation to circumvent the specific angiogenic blockade; and intrinsic or pre-existing indifference. Multiple mechanisms can be invoked in different tumour contexts to manifest both evasive and intrinsic resistance, motivating assessment of their prevalence and importance and in turn the design of pharmacological strategies that confer enduring anti-angiogenic therapies.
Figures


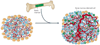

References
-
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nature Rev. Drug Discov. 2007;6:273–286. - PubMed
-
- Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005;333:328–335. - PubMed
-
- Jain RK. Antiangiogenic therapy for cancer: current and emerging concepts. Oncology (Williston Park) 2005;19:7–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

