Adaptation of endoplasmic reticulum exit sites to acute and chronic increases in cargo load
- PMID: 18650939
- PMCID: PMC2516884
- DOI: 10.1038/emboj.2008.136
Adaptation of endoplasmic reticulum exit sites to acute and chronic increases in cargo load
Abstract
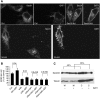
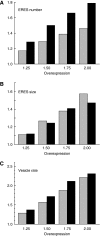
The biogenesis of endoplasmic reticulum (ER) exit sites (ERES) involves the formation of phosphatidylinositol-4 phosphate (PI4) and Sec16, but it is entirely unknown how ERES adapt to variations in cargo load. Here, we studied acute and chronic adaptive responses of ERES to an increase in cargo load for ER export. The acute response (within minutes) to increased cargo load stimulated ERES fusion events, leading to larger but less ERES. Silencing either PI4-kinase IIIalpha (PI4K-IIIalpha) or Sec16 inhibited the acute response. Overexpression of secretory cargo for 24 h induced the unfolded protein response (UPR), upregulated COPII, and the cells formed more ERES. This chronic response was insensitive to silencing PI4K-IIIalpha, but was abrogated by silencing Sec16. The UPR was required as the chronic response was absent in cells lacking inositol-requiring protein 1. Mathematical model simulations further support the notion that increasing ERES number together with COPII levels is an efficient way to enhance the secretory flux. These results indicate that chronic and acute increases in cargo load are handled differentially by ERES and are regulated by different factors.
Figures






References
-
- Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD (2007) XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27: 53–66 - PubMed
-
- Bevis BJ, Hammond AT, Reinke CA, Glick BS (2002) De novo formation of transitional ER sites and Golgi structures in Pichia pastoris. Nat Cell Biol 4: 750–756 - PubMed
-
- Bi X, Corpina RA, Goldberg J (2002) Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature 419: 271–277 - PubMed
-
- Blumental-Perry A, Haney CJ, Weixel KM, Watkins SC, Weisz OA, Aridor M (2006) Phosphatidylinositol 4-phosphate formation at ER exit sites regulates ER export. Dev Cell 11: 671–682 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

