Cloning of a newly identified heart-specific troponin I isoform, which lacks the troponin T binding portion, using the yeast hybrid system
- PMID: 18651010
- PMCID: PMC2274840
Cloning of a newly identified heart-specific troponin I isoform, which lacks the troponin T binding portion, using the yeast hybrid system
Abstract
Objective: To elucidate the molecular pathogenesis behind increased levels of laminin in cardiac muscle cells in cardiomyopathy by using a yeast hybrid screen. The present study reports the cloning of a newly identified heart-specific troponin I isoform, which is putatively linked to laminin. Future studies will explore the functional significance of this connection.
Methods: Yeast two-hybrid screen analysis was performed using MLF1-interacting protein (amino acids 1 to 318) as bait. The human heart complementary DNA library was screened by using the yeast-mating method for overnight culture.
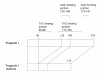
Results: Two final positive clones from the heart library were isolated. These two clones encoded the same protein, a short isoform of human cardiac troponin I (TnI) that lacked TnI exons 5 and 6. The TnI isoform has a heart-specific expression pattern and it shares several sequence features with human cardiac TnI; however, it lacks the troponin T binding portion.
Conclusion: The heart-specific segment of the human cardiac TnI isoform shares several sequence features with human cardiac TnI, but it lacks the troponin T binding portion. These results suggest that the heart-specific TnI isoform may be involved in cardiac development and disease.
Keywords: Hybrid system; TNNI3; Tropomyosin; Troponin I.
Figures



Similar articles
-
Tissue-specific alternative splicing of ascidian troponin I isoforms. Redesign of a protein isoform-generating mechanism during chordate evolution.J Biol Chem. 1997 Dec 19;272(51):32115-20. doi: 10.1074/jbc.272.51.32115. J Biol Chem. 1997. PMID: 9405409
-
Gene transfer of troponin I isoforms, mutants, and chimeras.Adv Exp Med Biol. 2003;538:169-74; discussion 174. doi: 10.1007/978-1-4419-9029-7_15. Adv Exp Med Biol. 2003. PMID: 15098664
-
An R111C polymorphism in wild turkey cardiac troponin I accompanying the dilated cardiomyopathy-related abnormal splicing variant of cardiac troponin T with potentially compensatory effects.J Biol Chem. 2004 Apr 2;279(14):13825-32. doi: 10.1074/jbc.M314225200. Epub 2004 Jan 20. J Biol Chem. 2004. PMID: 14736877
-
The miscommunicative cardiac cell: when good proteins go bad.Ann N Y Acad Sci. 2005 Jun;1047:30-7. doi: 10.1196/annals.1341.003. Ann N Y Acad Sci. 2005. PMID: 16093482 Review.
-
TNNI1, TNNI2 and TNNI3: Evolution, regulation, and protein structure-function relationships.Gene. 2016 Jan 15;576(1 Pt 3):385-94. doi: 10.1016/j.gene.2015.10.052. Epub 2015 Oct 23. Gene. 2016. PMID: 26526134 Free PMC article. Review.
Cited by
-
SARS-CoV-2 and Its Bacterial Co- or Super-Infections Synergize to Trigger COVID-19 Autoimmune Cardiopathies.Int J Mol Sci. 2023 Jul 29;24(15):12177. doi: 10.3390/ijms241512177. Int J Mol Sci. 2023. PMID: 37569555 Free PMC article.
References
-
- el-Saleh SC, Warber KD, Potter JD. The role of tropomyosin-troponin in the regulation of skeletal muscle contraction. J Muscle Res Cell Motil. 1986;7:387–404. - PubMed
-
- Bhavsar PK, Brand NJ, Yacoub MH, Barton PJ. Isolation and characterization of the human cardiac troponin I gene (TNNI3) Genomics. 1996;35:11–23. - PubMed
-
- Perry SV. Troponin I: Inhibitor or facilitator. Mol Cell Biochem. 1999;190:9–32. - PubMed
-
- Wilkinson JM, Grand RJ. Comparison of amino acid sequence of troponin I from different striated muscles. Nature. 1978;271:31–5. - PubMed
-
- Vallins WJ, Brand NJ, Dabhade N, Butler-Browne G, Yacoub MH, Barton PJ. Molecular cloning of human cardiac troponin I using polymerase chain reaction. FEBS Lett. 1990;270:57–61. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
