Synthesis of GABAA receptor agonists and evaluation of their alpha-subunit selectivity and orientation in the GABA binding site
- PMID: 18651727
- PMCID: PMC2566937
- DOI: 10.1021/jm701562x
Synthesis of GABAA receptor agonists and evaluation of their alpha-subunit selectivity and orientation in the GABA binding site
Abstract
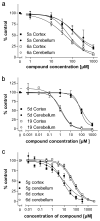
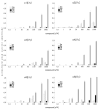
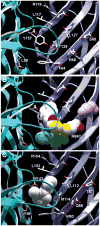
Drugs used to treat various disorders target GABA A receptors. To develop alpha subunit selective compounds, we synthesized 5-(4-piperidyl)-3-isoxazolol (4-PIOL) derivatives. The 3-isoxazolol moiety was substituted by 1,3,5-oxadiazol-2-one, 1,3,5-oxadiazol-2-thione, and substituted 1,2,4-triazol-3-ol heterocycles with modifications to the basic piperidine substituent as well as substituents without basic nitrogen. Compounds were screened by [(3)H]muscimol binding and in patch-clamp experiments with heterologously expressed GABA A alpha ibeta 3gamma 2 receptors (i = 1-6). The effects of 5-aminomethyl-3 H-[1,3,4]oxadiazol-2-one 5d were comparable to GABA for all alpha subunit isoforms. 5-piperidin-4-yl-3 H-[1,3,4]oxadiazol-2-one 5a and 5-piperidin-4-yl-3 H-[1,3,4]oxadiazol-2-thione 6a were weak agonists at alpha 2-, alpha 3-, and alpha 5-containing receptors. When coapplied with GABA, they were antagonistic in alpha 2-, alpha 4-, and alpha 6-containing receptors and potentiated alpha 3-containing receptors. 6a protected GABA binding site cysteine-substitution mutants alpha 1F64C and alpha 1S68C from reacting with methanethiosulfonate-ethylsulfonate. 6a specifically covalently modified the alpha 1R66C thiol, in the GABA binding site, through its oxadiazolethione sulfur. These results demonstrate the feasibility of synthesizing alpha subtype selective GABA mimetic drugs.
Figures











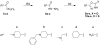
References
-
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50(2):291–313. - PubMed
-
- Böhme I, Rabe H, Lüddens H. Four amino acids in the alpha subunits determine the gamma-aminobutyric acid sensitivities of GABAA receptor subtypes. J Biol Chem. 2004;279(34):35193–200. - PubMed
-
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2(8):795–816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

