Engineering of xylose reductase and overexpression of xylitol dehydrogenase and xylulokinase improves xylose alcoholic fermentation in the thermotolerant yeast Hansenula polymorpha
- PMID: 18651968
- PMCID: PMC2515283
- DOI: 10.1186/1475-2859-7-21
Engineering of xylose reductase and overexpression of xylitol dehydrogenase and xylulokinase improves xylose alcoholic fermentation in the thermotolerant yeast Hansenula polymorpha
Abstract
Background: The thermotolerant methylotrophic yeast Hansenula polymorpha is capable of alcoholic fermentation of xylose at elevated temperatures (45 - 48 degrees C). Such property of this yeast defines it as a good candidate for the development of an efficient process for simultaneous saccharification and fermentation. However, to be economically viable, the main characteristics of xylose fermentation of H. polymorpha have to be improved.
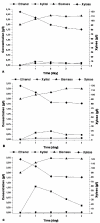
Results: Site-specific mutagenesis of H. polymorpha XYL1 gene encoding xylose reductase was carried out to decrease affinity of this enzyme toward NADPH. The modified version of XYL1 gene under control of the strong constitutive HpGAP promoter was overexpressed on a Deltaxyl1 background. This resulted in significant increase in the KM for NADPH in the mutated xylose reductase (K341 --> R N343 --> D), while KM for NADH remained nearly unchanged. The recombinant H. polymorpha strain overexpressing the mutated enzyme together with native xylitol dehydrogenase and xylulokinase on Deltaxyl1 background was constructed. Xylose consumption, ethanol and xylitol production by the constructed strain were determined for high-temperature xylose fermentation at 48 degrees C. A significant increase in ethanol productivity (up to 7.3 times) was shown in this recombinant strain as compared with the wild type strain. Moreover, the xylitol production by the recombinant strain was reduced considerably to 0.9 mg x (L x h)-1 as compared to 4.2 mg x (L x h)-1 for the wild type strain.
Conclusion: Recombinant strains of H. polymorpha engineered for improved xylose utilization are described in the present work. These strains show a significant increase in ethanol productivity with simultaneous reduction in the production of xylitol during high-temperature xylose fermentation.
Figures

References
-
- Zaldivar J, Nielsen J, Olsson L. Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl Microbiol Biotechnol. 2001;56:17–34. - PubMed
-
- Chiang C, Knight SC. Metabolism of D-xylose by molds. Nature. 1960;188:79–81. - PubMed
-
- Tantirungkij M, Nakashima N, Seki T, Yoshida T. Construction of xylose-assimilating Saccharomyces cerevisiae. J Ferment Bioeng. 1993;75:83–88.
-
- Toivari MH, Aristidou A, Ruohonen L, Penttila M. Conversion of xylose to ethanol by recombinant Saccharomyces cerevisiae: importance of xylulokinase (XKS1) and oxygen availability. Metab Eng. 2001;3:236–249. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

