Mitochondrial-mediated suppression of ROS production upon exposure of neurons to lethal stress: mitochondrial targeted preconditioning
- PMID: 18652858
- PMCID: PMC2612561
- DOI: 10.1016/j.addr.2008.03.020
Mitochondrial-mediated suppression of ROS production upon exposure of neurons to lethal stress: mitochondrial targeted preconditioning
Abstract
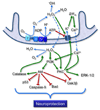
Preconditioning represents the condition where transient exposure of cells to an initiating event leads to protection against subsequent, potentially lethal stimuli. Recent studies have established that mitochondrial-centered mechanisms are important mediators in promoting development of the preconditioning response. However, many details concerning these mechanisms are unclear. The purpose of this review is to describe the initiating and subsequent intracellular events involving mitochondria which can lead to neuronal preconditioning. These mitochondrial specific targets include: 1) potassium channels located on the inner mitochondrial membrane; 2) respiratory chain enzymes; and 3) oxidative phosphorylation. Following activation of mitochondrial ATP-sensitive potassium (mitoK(ATP)) channels and/or increased production of reactive oxygen species (ROS) resulting from the disruption of the respiratory chain or during energy substrate deprivation, morphological changes or signaling events involving protein kinases confer immediate or delayed preconditioning on neurons that will allow them to survive otherwise lethal insults. While the mechanisms involved are not known with certainty, the results of preconditioning are the enhanced neuronal viability, the attenuated influx of intracellular calcium, the reduced availability of ROS, the suppression of apoptosis, and the maintenance of ATP levels during and following stress.
Figures
References
-
- Perez-Pinzon MA, Dave KR, Raval AP. Role of reactive oxygen species and protein kinase C in ischemic tolerance in the brain. Antioxid. Redox. Signal. 2005;7:1150–1157. - PubMed
-
- Duchen MR. Roles of mitochondria in health and disease. Diabetes. 2004;53 Suppl 1:S96–S102. - PubMed
-
- Kirino T. Ischemic tolerance. J. Cereb. Blood Flow Metab. 2002;22:1283–1296. - PubMed
-
- Gidday JM JM. Cerebral preconditioning and ischaemic tolerance. Nat. Rev. Neurosci. 2006;7:437–448. - PubMed
-
- Kis B, Rajapakse N, Snipes JA, Nagy K, Horiguchi T, Busija DW. Diazoxide induces delayed preconditioning in cultured rat cortical neurons. J. Neurochem. 2003;87:969–980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

