Monoamine oxidase inactivation: from pathophysiology to therapeutics
- PMID: 18652859
- PMCID: PMC2630537
- DOI: 10.1016/j.addr.2008.06.002
Monoamine oxidase inactivation: from pathophysiology to therapeutics
Abstract
Monoamine oxidases (MAOs) A and B are mitochondrial bound isoenzymes which catalyze the oxidative deamination of dietary amines and monoamine neurotransmitters, such as serotonin, norepinephrine, dopamine, beta-phenylethylamine and other trace amines. The rapid degradation of these molecules ensures the proper functioning of synaptic neurotransmission and is critically important for the regulation of emotional behaviors and other brain functions. The byproducts of MAO-mediated reactions include several chemical species with neurotoxic potential, such as hydrogen peroxide, ammonia and aldehydes. As a consequence, it is widely speculated that prolonged excessive activity of these enzymes may be conducive to mitochondrial damages and neurodegenerative disturbances. In keeping with these premises, the development of MAO inhibitors has led to important breakthroughs in the therapy of several neuropsychiatric disorders, ranging from mood disorders to Parkinson's disease. Furthermore, the characterization of MAO knockout (KO) mice has revealed that the inactivation of this enzyme produces a number of functional and behavioral alterations, some of which may be harnessed for therapeutic aims. In this article, we discuss the intriguing hypothesis that the attenuation of the oxidative stress induced by the inactivation of either MAO isoform may contribute to both antidepressant and antiparkinsonian actions of MAO inhibitors. This possibility further highlights MAO inactivation as a rich source of novel avenues in the treatment of mental disorders.
Figures
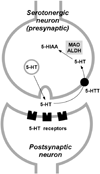
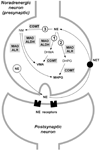


References
-
- Johnston JP. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem. Pharmacol. 1968;17:1285–1297. - PubMed
-
- Knoll J, Magyar K. Some puzzling pharmacological effects of monoamine oxidase inhibitors. Adv. Biochem. Psychopharmacol. 1972;5:393–408. - PubMed
-
- Lan NC, Heinzmann C, Gal A, Klisak I, Orth U, Lai E, Grimsby J, Sparkes RS, Mohandas T, Shih JC. Human monoamine oxidase A and B genes map to Xp 11.23 and are deleted in a patient with norrie disease. Genomics. 1989;4:552–559. - PubMed
-
- Shih JC, Chen K, Geha RM. Determination of regions important for monoamine oxidase (mao) a and b substrate and inhibitor selectivities. J. Neural Transm. Suppl. 1998;52:1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

