Inflammatory demyelination induces axonal injury and retinal ganglion cell apoptosis in experimental optic neuritis
- PMID: 18653182
- PMCID: PMC2564281
- DOI: 10.1016/j.exer.2008.05.017
Inflammatory demyelination induces axonal injury and retinal ganglion cell apoptosis in experimental optic neuritis
Abstract
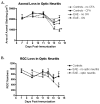
Optic neuritis is an inflammatory disease of the optic nerve that often occurs in patients with multiple sclerosis and leads to permanent visual loss mediated by retinal ganglion cell (RGC) damage. Optic neuritis occurs with high frequency in relapsing-remitting experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis, with significant loss of RGCs. In the current study, mechanisms of RGC loss in this model were examined to determine whether inflammation-induced axonal injury mediates apoptotic death of RGCs. RGCs were retrogradely labeled by injection of fluorogold into superior colliculi of 6-7 week old female SJL/J mice. EAE was induced one week later by immunization with proteolipid protein peptide. Optic neuritis was detected by inflammatory cell infiltration on histological examination as early as 9 days after immunization, with peak incidence by day 12. Demyelination occurred 1-2 days after inflammation began. Loss of RGC axons was detected following demyelination, with significant axonal loss occurring by day 13 post-immunization. Axonal loss occurred prior to loss of RGC bodies at day 14. Apoptotic cells were also observed at day 14 in the ganglion cell layer of eyes with optic neuritis, but not in control eyes. Together these results suggest that inflammatory cell infiltration mediates demyelination and leads to direct axonal injury in this model of experimental optic neuritis. RGCs die by an apoptotic mechanism triggered by axonal injury. Potential neuroprotective therapies to prevent permanent RGC loss from optic neuritis will likely need to be initiated prior to axonal injury to preserve neuronal function.
Figures




References
-
- Arnold AC. Evolving management of optic neuritis and multiple sclerosis. Am J Ophthalmol. 2005;139:1101–1108. - PubMed
-
- Beck RW, Cleary PA, Anderson MM, Jr, Keltner JL, Shults WT, Kaufman DI, Buckley EG, Corbett JJ, Kupersmith MJ, Miller NR, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. New Eng J Med. 1992;326:581–588. - PubMed
-
- Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, Freedman MS, Zackon DH, Kardon RH. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963–969. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

