Vascular endothelial growth factor in eye disease
- PMID: 18653375
- PMCID: PMC3682685
- DOI: 10.1016/j.preteyeres.2008.05.001
Vascular endothelial growth factor in eye disease
Abstract
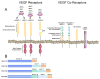
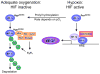
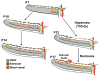
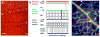
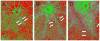
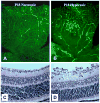
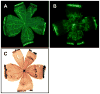
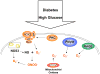
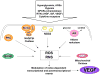
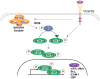
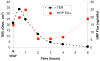
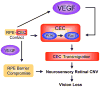
Collectively, angiogenic ocular conditions represent the leading cause of irreversible vision loss in developed countries. In the US, for example, retinopathy of prematurity, diabetic retinopathy and age-related macular degeneration are the principal causes of blindness in the infant, working age and elderly populations, respectively. Evidence suggests that vascular endothelial growth factor (VEGF), a 40kDa dimeric glycoprotein, promotes angiogenesis in each of these conditions, making it a highly significant therapeutic target. However, VEGF is pleiotropic, affecting a broad spectrum of endothelial, neuronal and glial behaviors, and confounding the validity of anti-VEGF strategies, particularly under chronic disease conditions. In fact, among other functions VEGF can influence cell proliferation, cell migration, proteolysis, cell survival and vessel permeability in a wide variety of biological contexts. This article will describe the roles played by VEGF in the pathogenesis of retinopathy of prematurity, diabetic retinopathy and age-related macular degeneration. The potential disadvantages of inhibiting VEGF will be discussed, as will the rationales for targeting other VEGF-related modulators of angiogenesis.
Figures
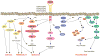





References
-
- Abu El-Asrar AM, Meersschaert A, et al. Inducible nitric oxide synthase and vascular endothelial growth factor are colocalized in the retinas of human subjects with diabetes. Eye. 2004;18:306–313. - PubMed
-
- Adamis AP, Miller JW, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450. - PubMed
-
- Adamis AP, Shima DT, et al. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun. 1993;193:631–638. - PubMed
-
- Adamis AP, Shima DT, et al. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol. 1996;114:66–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY004618/EY/NEI NIH HHS/United States
- R56 EY015130/EY/NEI NIH HHS/United States
- R01 EY015130/EY/NEI NIH HHS/United States
- EY015130/EY/NEI NIH HHS/United States
- R01 EY011766/EY/NEI NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- EY011766/EY/NEI NIH HHS/United States
- R01 EY017011/EY/NEI NIH HHS/United States
- T32 EY007135/EY/NEI NIH HHS/United States
- EY007533/EY/NEI NIH HHS/United States
- R01 EY007533/EY/NEI NIH HHS/United States
- EY04618/EY/NEI NIH HHS/United States
- EY07135/EY/NEI NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- EY017011/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

