Review. Neurobiological mechanisms for opponent motivational processes in addiction
- PMID: 18653439
- PMCID: PMC2607326
- DOI: 10.1098/rstb.2008.0094
Review. Neurobiological mechanisms for opponent motivational processes in addiction
Abstract
The conceptualization of drug addiction as a compulsive disorder with excessive drug intake and loss of control over intake requires motivational mechanisms. Opponent process as a motivational theory for the negative reinforcement of drug dependence has long required a neurobiological explanation. Key neurochemical elements involved in reward and stress within basal forebrain structures involving the ventral striatum and extended amygdala are hypothesized to be dysregulated in addiction to convey the opponent motivational processes that drive dependence. Specific neurochemical elements in these structures include not only decreases in reward neurotransmission such as dopamine and opioid peptides in the ventral striatum, but also recruitment of brain stress systems such as corticotropin-releasing factor (CRF), noradrenaline and dynorphin in the extended amygdala. Acute withdrawal from all major drugs of abuse produces increases in reward thresholds, anxiety-like responses and extracellular levels of CRF in the central nucleus of the amygdala. CRF receptor antagonists block excessive drug intake produced by dependence. A brain stress response system is hypothesized to be activated by acute excessive drug intake, to be sensitized during repeated withdrawal, to persist into protracted abstinence and to contribute to stress-induced relapse. The combination of loss of reward function and recruitment of brain stress systems provides a powerful neurochemical basis for the long hypothesized opponent motivational processes responsible for the negative reinforcement driving addiction.
Figures

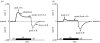
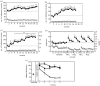


References
-
- Ahmed S.H, Koob G.F. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi:10.1126/science.282.5387.298 - DOI - PubMed
-
- Ahmed S.H, Walker J.R, Koob G.F. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi:10.1016/S0893-133X(99)00133-5 - DOI - PubMed
-
- Alheid G.F, De Olmos J.S, Beltramino C.A. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system. Academic Press; San Diego, CA: 1995. pp. 495–578.
-
- American Psychiatric Association. 4th edn. American Psychiatric Press; Washington, DC: 1994. Diagnostic and statistical manual of mental disorders.
-
- Aston-Jones, G., Delfs, J. M., Druhan, J. & Zhu, Y. 1999 The bed nucleus of the stria terminalis: a target site for noradrenergic actions in opiate withdrawal. In Advancing from the ventral striatum to the extended amygdala: implications for neuropsychiatry and drug abuse, vol. 877 (ed. J. F. McGinty). Annals of the New York Academy of Sciences, pp. 486–498. New York, NY: New York Academy of Sciences. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA10072/DA/NIDA NIH HHS/United States
- DK26741/DK/NIDDK NIH HHS/United States
- DA04398/DA/NIDA NIH HHS/United States
- AA08459/AA/NIAAA NIH HHS/United States
- R01 AA008459/AA/NIAAA NIH HHS/United States
- P01 DK026741/DK/NIDDK NIH HHS/United States
- DA04043/DA/NIDA NIH HHS/United States
- R01 DA004043/DA/NIDA NIH HHS/United States
- R01 DA004398/DA/NIDA NIH HHS/United States
- P50 AA006420/AA/NIAAA NIH HHS/United States
- AA06420/AA/NIAAA NIH HHS/United States
- R37 AA008459/AA/NIAAA NIH HHS/United States
- R01 DA010072/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
