Poliovirus cis-acting replication element-dependent VPg Uridylylation lowers the Km of the initiating nucleoside triphosphate for viral RNA replication
- PMID: 18653453
- PMCID: PMC2546976
- DOI: 10.1128/JVI.00427-08
Poliovirus cis-acting replication element-dependent VPg Uridylylation lowers the Km of the initiating nucleoside triphosphate for viral RNA replication
Abstract
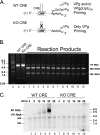
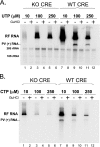
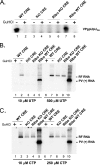
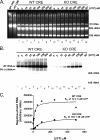
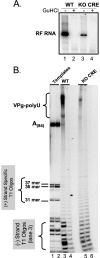
Initiation of RNA synthesis by RNA-dependent RNA polymerases occurs when a phosphodiester bond is formed between the first two nucleotides in the 5' terminus of product RNA. The concentration of initiating nucleoside triphosphates (NTPi) required for RNA synthesis is typically greater than the concentration of NTPs required for elongation. VPg, a small viral protein, is covalently attached to the 5' end of picornavirus negative- and positive-strand RNAs. A cis-acting replication element (CRE) within picornavirus RNAs serves as a template for the uridylylation of VPg, resulting in the synthesis of VPgpUpU(OH). Mutations within the CRE RNA structure prevent VPg uridylylation. While the tyrosine hydroxyl of VPg can prime negative-strand RNA synthesis in a CRE- and VPgpUpU(OH)-independent manner, CRE-dependent VPgpUpU(OH) synthesis is absolutely required for positive-strand RNA synthesis. As reported herein, low concentrations of UTP did not support negative-strand RNA synthesis when CRE-disrupting mutations prevented VPg uridylylation, whereas correspondingly low concentrations of CTP or GTP had no negative effects on the magnitude of CRE-independent negative-strand RNA synthesis. The experimental data indicate that CRE-dependent VPg uridylylation lowers the K(m) of UTP required for viral RNA replication and that CRE-dependent VPgpUpU(OH) synthesis was required for efficient negative-strand RNA synthesis, especially when UTP concentrations were limiting. By lowering the concentration of UTP needed for the initiation of RNA replication, CRE-dependent VPg uridylylation provides a mechanism for a more robust initiation of RNA replication.
Figures
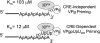
References
-
- Agol, V. I., A. V. Paul, and E. Wimmer. 1999. Paradoxes of the replication of picornaviral genomes. Virus Res. 62129-147. - PubMed
-
- Amiott, E. A., and J. A. Jaehning. 2006. Mitochondrial transcription is regulated via an ATP “sensing” mechanism that couples RNA abundance to respiration. Mol. Cell 22329-338. - PubMed
-
- Amiott, E. A., and J. A. Jaehning. 2006. Sensitivity of the yeast mitochondrial RNA polymerase to +1 and +2 initiating nucleotides. J. Biol. Chem. 28134982-34988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

