Systems analysis of the single photon response in invertebrate photoreceptors
- PMID: 18653755
- PMCID: PMC2492478
- DOI: 10.1073/pnas.0711884105
Systems analysis of the single photon response in invertebrate photoreceptors
Abstract
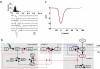
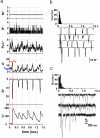
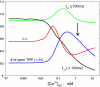
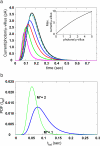
Photoreceptors of Drosophila compound eye employ a G protein-mediated signaling pathway that transduces single photons into transient electrical responses called "quantum bumps" (QB). Although most of the molecular components of this pathway are already known, the system-level understanding of the mechanism of QB generation has remained elusive. Here, we present a quantitative model explaining how QBs emerge from stochastic nonlinear dynamics of the signaling cascade. The model shows that the cascade acts as an "integrate and fire" device and explains how photoreceptors achieve reliable responses to light although keeping low background in the dark. The model predicts the nontrivial behavior of mutants that enhance or suppress signaling and explains the dependence on external calcium, which controls feedback regulation. The results provide insight into physiological questions such as single-photon response efficiency and the adaptation of response to high incident-light level. The system-level analysis enabled by modeling phototransduction provides a foundation for understanding G protein signaling pathways less amenable to quantitative approaches.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–193. - PubMed
-
- Hardie RC, et al. Molecular basis of amplification in Drosophila phototransduction: Roles for G protein, phospholipase C, and diacylglycerol kinase. Neuron. 2002;36:689–701. - PubMed
-
- Hardie RC. TRP channels in Drosophila photoreceptors: the lipid connection. Cell Calcium. 2003;33:385–393. - PubMed
-
- Ranganathan R, Malicki DM, Zuker CS. Signal transduction in Drosophila photoreceptors. Annu Rev Neurosci. 1995;18:283–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

