Native 3D intermediates of membrane fusion in herpes simplex virus 1 entry
- PMID: 18653756
- PMCID: PMC2492464
- DOI: 10.1073/pnas.0801674105
Native 3D intermediates of membrane fusion in herpes simplex virus 1 entry
Abstract
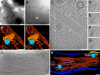
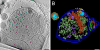
The concerted action of four viral glycoproteins and at least one cellular receptor is required to catalyze herpes simplex virus 1 entry into host cells either by fusion at the plasma membrane or intracellularly after internalization by endocytosis. Here, we applied cryo electron tomography to capture 3D intermediates from Herpes simplex virus 1 fusion at the plasma membrane in their native environment by using two model systems: adherent cells and synaptosomes. The fusion process was delineated as a series of structurally different steps. The incoming capsid separated from the tegument and was closely surrounded by the cortical cytoskeleton. After entry, the viral membrane curvature changed concomitantly with a reorganization of the envelope glycoprotein spikes. Individual glycoprotein complexes in transitional conformations during pore formation and dilation revealed the complex viral fusion mechanism in action. Snapshots of the fusion intermediates provide unprecedented details concerning the overall structural changes occurring during herpesvirus entry. Moreover, our data suggest that there are two functional "poles" of the asymmetric herpesvirion: one related to cell entry, and the other formed during virus assembly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

