VDAC regulation: role of cytosolic proteins and mitochondrial lipids
- PMID: 18654841
- PMCID: PMC2671000
- DOI: 10.1007/s10863-008-9145-y
VDAC regulation: role of cytosolic proteins and mitochondrial lipids
Abstract
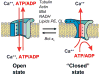
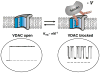
It was recently asserted that the voltage-dependent anion channel (VDAC) serves as a global regulator, or governor, of mitochondrial function (Lemasters and Holmuhamedov, Biochim Biophys Acta 1762:181-190, 2006). Indeed, VDAC, positioned on the interface between mitochondria and the cytosol (Colombini, Mol Cell Biochem 256:107-115, 2004), is at the control point of mitochondria life and death. This large channel plays the role of a "switch" that defines in which direction mitochondria will go: to normal respiration or to suppression of mitochondria metabolism that leads to apoptosis and cell death. As the most abundant protein in the mitochondrial outer membrane (MOM), VDAC is known to be responsible for ATP/ADP exchange and for the fluxes of other metabolites across MOM. It controls them by switching between the open and "closed" states that are virtually impermeable to ATP and ADP. This control has dual importance: in maintaining normal mitochondria respiration and in triggering apoptosis when cytochrome c and other apoptogenic factors are released from the intermembrane space into the cytosol. Emerging evidence indicates that VDAC closure promotes apoptotic signals without direct involvement of VDAC in the permeability transition pore or hypothetical Bax-containing cytochrome c permeable pores. VDAC gating has been studied extensively for the last 30 years on reconstituted VDAC channels. In this review we focus exclusively on physiologically relevant regulators of VDAC gating such as endogenous cytosolic proteins and mitochondrial lipids. Closure of VDAC induced by such dissimilar cytosolic proteins as pro-apoptotic tBid and dimeric tubulin is compared to show that the involved mechanisms are rather distinct. While tBid mostly modulates VDAC voltage gating, tubulin blocks the channel with the efficiency of blockage controlled by voltage. We also discuss how characteristic mitochondrial lipids, phospatidylethanolamine and cardiolipin, could regulate VDAC gating. Overall, we demonstrate that VDAC gating is not just an observation made under artificial conditions of channel reconstitution but is a major mechanism of MOM permeability control.
Figures

References
-
- Andre N, Carre M, Brasseur G, Pourroy B, Kovacic H, Briand C, Braguer D. FEBS Lett. 2002;532:256–260. - PubMed
-
- Appaix F, Kuznetsov AV, Usson Y, Kay L, Andrienko T, Olivares J, Kaambre T, Sikk P, Margreiter R, Saks V. Exp Physiol. 2003;88:175–190. - PubMed
-
- Ardail D, Privat JP, Egretcharlier M, Levrat C, Lerme F, Louisot P. J Biol Chem. 1990;265:18797–18802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

