Role of lateral hypothalamic orexin neurons in reward processing and addiction
- PMID: 18655797
- PMCID: PMC2635332
- DOI: 10.1016/j.neuropharm.2008.06.060
Role of lateral hypothalamic orexin neurons in reward processing and addiction
Abstract
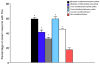
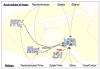
Orexins (also known as hypocretins) are recently discovered neuropeptides made exclusively in hypothalamic neurons that have been shown to be important in narcolepsy/cataplexy and arousal. Here, we conducted behavioral, anatomical and neurophysiological studies that show that a subset of these cells, located specifically in lateral hypothalamus (LH), are involved in reward processing and addictive behaviors. We found that Fos expression in LH orexin neurons varied in proportion to preference for morphine, cocaine or food. This relationship obtained both in drug naïve rats and in animals during protracted morphine withdrawal, when drug preference was elevated but food preference was decreased. Recent studies showed that LH orexin neurons that project to ventral tegmental area (VTA) have greater Fos induction in association with elevated morphine preference during protracted withdrawal than non-VTA-projecting orexin neurons, indicating that the VTA is an important site of action for orexin's role in reward processing. In addition, we found that stimulation of LH orexin neurons, or microinjection of orexin into VTA, reinstated an extinguished morphine preference. Most recently, using a self-administration paradigm we discovered that the Ox1 receptor antagonist SB-334867 (SB) blocks cocaine-seeking induced by discrete or contextual cues, but not by a priming injection of cocaine. Neurophysiological studies revealed that locally applied orexin often augmented responses of VTA dopamine (DA) neurons to activation of the medial prefrontal cortex (mPFC), consistent with the view that orexin facilitates activation of VTA DA neurons by stimulus-reward associations. We also recently showed that orexin in VTA is necessary for learning a morphine place preference. These findings are consistent with results from others showing that orexin facilitates glutamate-mediated responses, and is necessary for glutamate-dependent long-term potentiation, in VTA DA neurons. We surmise from these studies that LH orexin neurons play an important role in reward processing and addiction, and that LH orexin cells are an important input to VTA for behavioral effects associated with reward-paired stimuli.
Figures




References
-
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47S1:167–179. - PubMed
-
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

