The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT)
- PMID: 18656454
- PMCID: PMC2748879
- DOI: 10.1016/j.bcp.2008.06.022
The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT)
Abstract
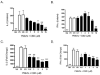
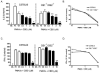
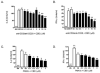
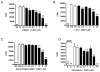
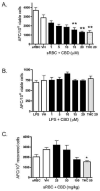
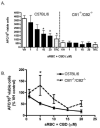
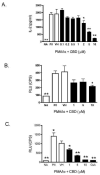
Cannabidiol (CBD) is a cannabinoid compound derived from Cannabis Sativa that does not possess high affinity for either the CB1 or CB2 cannabinoid receptors. Similar to other cannabinoids, we demonstrated previously that CBD suppressed interleukin-2 (IL-2) production from phorbol ester plus calcium ionophore (PMA/Io)-activated murine splenocytes. Thus, the focus of the present studies was to further characterize the effect of CBD on immune function. CBD also suppressed IL-2 and interferon-gamma (IFN-gamma) mRNA expression, proliferation, and cell surface expression of the IL-2 receptor alpha chain, CD25. While all of these observations support the fact that CBD suppresses T cell function, we now demonstrate that CBD suppressed IL-2 and IFN-gamma production in purified splenic T cells. CBD also suppressed activator protein-1 (AP-1) and nuclear factor of activated T cells (NFAT) transcriptional activity, which are critical regulators of IL-2 and IFN-gamma. Furthermore, CBD suppressed the T cell-dependent anti-sheep red blood cell immunoglobulin M antibody forming cell (anti-sRBC IgM AFC) response. Finally, using splenocytes derived from CB1(-/-)/CB2(-/-) mice, it was determined that suppression of IL-2 and IFN-gamma and suppression of the in vitro anti-sRBC IgM AFC response occurred independently of both CB1 and CB2. However, the magnitude of the immune response to sRBC was significantly depressed in CB1(-/-)/CB2(-/-) mice. Taken together, these data suggest that CBD suppresses T cell function and that CB1 and/or CB2 play a critical role in the magnitude of the in vitro anti-sRBC IgM AFC response.
Figures

Similar articles
-
2-Arachidonoyl-glycerol suppresses interferon-gamma production in phorbol ester/ionomycin-activated mouse splenocytes independent of CB1 or CB2.J Leukoc Biol. 2005 Jun;77(6):966-74. doi: 10.1189/jlb.1104652. Epub 2005 Mar 17. J Leukoc Biol. 2005. PMID: 15774549
-
Effects of targeted deletion of cannabinoid receptors CB1 and CB2 on immune competence and sensitivity to immune modulation by Delta9-tetrahydrocannabinol.J Leukoc Biol. 2008 Dec;84(6):1574-84. doi: 10.1189/jlb.0508282. Epub 2008 Sep 12. J Leukoc Biol. 2008. PMID: 18791168 Free PMC article.
-
Suppressive effects of cannabidiol on antigen-specific antibody production and functional activity of splenocytes in ovalbumin-sensitized BALB/c mice.Int Immunopharmacol. 2007 Jun;7(6):773-80. doi: 10.1016/j.intimp.2007.01.015. Epub 2007 Feb 15. Int Immunopharmacol. 2007. PMID: 17466911
-
The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin.Br J Pharmacol. 2008 Jan;153(2):199-215. doi: 10.1038/sj.bjp.0707442. Epub 2007 Sep 10. Br J Pharmacol. 2008. PMID: 17828291 Free PMC article. Review.
-
Cannabidiol and periodontal inflammatory disease: A critical assessment.Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2022 May;166(2):155-160. doi: 10.5507/bp.2022.012. Epub 2022 Mar 21. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2022. PMID: 35332345 Review.
Cited by
-
Immune Responses Regulated by Cannabidiol.Cannabis Cannabinoid Res. 2020 Feb 27;5(1):12-31. doi: 10.1089/can.2018.0073. eCollection 2020 Mar 1. Cannabis Cannabinoid Res. 2020. PMID: 32322673 Free PMC article. Review.
-
Utilization of Cannabidiol in Post-Organ-Transplant Care.Int J Mol Sci. 2025 Jan 15;26(2):699. doi: 10.3390/ijms26020699. Int J Mol Sci. 2025. PMID: 39859413 Free PMC article. Review.
-
An open-label phase I comparator-controlled clinical trial to assess tolerability and pharmacokinetics of IHL-675 A a fixed dose combination of cannabidiol plus hydroxychloroquine in healthy volunteers.Sci Rep. 2025 Jun 3;15(1):19357. doi: 10.1038/s41598-025-04573-5. Sci Rep. 2025. PMID: 40456820 Free PMC article. Clinical Trial.
-
Critical Role of Mast Cells and Peroxisome Proliferator-Activated Receptor γ in the Induction of Myeloid-Derived Suppressor Cells by Marijuana Cannabidiol In Vivo.J Immunol. 2015 Jun 1;194(11):5211-22. doi: 10.4049/jimmunol.1401844. Epub 2015 Apr 27. J Immunol. 2015. PMID: 25917103 Free PMC article.
-
Targeting natural products against SARS-CoV-2.Environ Sci Pollut Res Int. 2022 Jun;29(28):42404-42432. doi: 10.1007/s11356-022-19770-2. Epub 2022 Apr 1. Environ Sci Pollut Res Int. 2022. PMID: 35362883 Free PMC article. Review.
References
-
- Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86(8):1646–1647.
-
- Matsuda LA, Lolait SJ. Brownstein MJ, Young AC and Bonner TI, Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature (London) 1990;346:561–564. - PubMed
-
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature (London) 1993;365:61–65. - PubMed
-
- Srivastava MD, Srivastava BI, Brouhard B. Delta9 tetrahydrocannabinol and cannabidiol alter cytokine production by human immune cells. Immunopharmacology. 1998;40(3):179–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

