Purification and mutagenesis of LpxL, the lauroyltransferase of Escherichia coli lipid A biosynthesis
- PMID: 18656959
- PMCID: PMC2664092
- DOI: 10.1021/bi800873n
Purification and mutagenesis of LpxL, the lauroyltransferase of Escherichia coli lipid A biosynthesis
Abstract
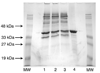
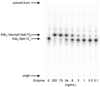
Escherichia coli lipid A is a hexaacylated disaccharide of glucosamine with secondary laurate and myristate chains on the distal unit. Hexaacylated lipid A is a potent agonist of human Toll-like receptor 4, whereas its tetra- and pentaacylated precursors are antagonists. The inner membrane enzyme LpxL transfers laurate from lauroyl-acyl carrier protein to the 2'- R-3-hydroxymyristate moiety of the tetraacylated lipid A precursor Kdo 2-lipid IV A. LpxL has now been overexpressed, solubilized with n-dodecyl beta- d-maltopyranoside (DDM), and purified to homogeneity. LpxL migration on a gel filtration column is consistent with a molecular mass of 80 kDa, suggestive of an LpxL monomer (36 kDa) embedded in a DDM micelle. Mass spectrometry showed that deformylated LpxL was the predominant species, noncovalently bound to as many as 12 DDM molecules. Purified LpxL catalyzed not only the formation in vitro of Kdo 2-(lauroyl)-lipid IV A but also a slow second acylation, generating Kdo 2-(dilauroyl)-lipid IV A. Consistent with the Kdo dependence of crude LpxL in membranes, Kdo 2-lipid IV A is preferred 6000-fold over lipid IV A by the pure enzyme. Sequence comparisons suggest that LpxL shares distant homology with the glycerol-3-phosphate acyltransferase (GPAT) family, including a putative catalytic dyad located in a conserved H(X) 4D/E motif. Mutation of H132 or E137 to alanine reduces specific activity by over 3 orders of magnitude. Like many GPATs, LpxL can also utilize acyl-CoA as an alternative acyl donor, albeit at a slower rate. Our results show that the acyltransferases that generate the secondary acyl chains of lipid A are members of the GPAT family and set the stage for structural studies.
Figures







References
-
- Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 2005;3:36–46. - PubMed
-
- Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, Lee JO. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–917. - PubMed
-
- Coats SR, Do CT, Karimi-Naser LM, Braham PH, Darveau RP. Antagonistic lipopolysaccharides block E. coli lipopolysaccharide function at human TLR4 via interaction with the human MD-2 lipopolysaccharide binding site. Cell Microbiol. 2007;9:1191–1202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

