Interleukin 8 is differently expressed and modulated by PAR-1 activation in early and late endothelial progenitor cells
- PMID: 18657231
- PMCID: PMC6512392
- DOI: 10.1111/j.1582-4934.2008.00429.x
Interleukin 8 is differently expressed and modulated by PAR-1 activation in early and late endothelial progenitor cells
Abstract
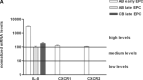
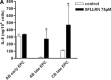
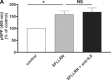
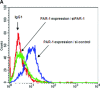
The proinflammatory chemokine interleukin 8 exerts potent angiogenic effects on endothelial cells by interacting with its receptors CXCR1 and CXCR2. As thrombin is also a potent inflammatory factor, and as endothelial progenitor cells (EPC) express functional PAR-1 thrombin receptor, we examined whether PAR-1 stimulation interferes with the IL-8 pathway in EPC. EPC were obtained from adult blood (AB) and cord blood (CB). The effect of PAR-1 stimulation by the peptide SFLLRN on IL-8, CXCR1 and CXCR2 expression was examined by RTQ-PCR and at the protein level in AB and CB late EPC and in AB early EPC. Specific siRNA was used to knock down PAR-1 expression. The IL-8 gene was expressed strongly in AB early EPC and moderately in late EPC. In contrast, CXCR1 and CXCR2 gene expression was restricted to AB early EPC. The IL-8 level in AB early EPC conditioned medium was high in basal conditions and did not change after PAR-1 activation. By contrast, IL-8 secretion by late EPC was low in basal conditions and strongly up-regulated upon PAR-1 activation. PAR-1 activation induced a number of genes involved in activating protein-1 (AP-1) and nuclear factor (NF)-kappaB pathways. Conditioned medium of PAR-1-activated late EPC enhanced the migratory potential of early EPC, and this effect was abrogated by blocking IL-8. Target-specific siRNA-induced PAR-1 knockdown, and fully inhibited PAR-1-induced IL-8 synthesis. In conclusion, PAR-1 activation induces IL-8 synthesis by late EPC. This could potentially enhance cooperation between late and early EPC during neovascularization, through a paracrine effect.
Figures

















Similar articles
-
PAR-1 activation on human late endothelial progenitor cells enhances angiogenesis in vitro with upregulation of the SDF-1/CXCR4 system.Arterioscler Thromb Vasc Biol. 2005 Nov;25(11):2321-7. doi: 10.1161/01.ATV.0000184762.63888.bd. Epub 2005 Sep 1. Arterioscler Thromb Vasc Biol. 2005. PMID: 16141404
-
Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2.J Biol Chem. 2003 Mar 7;278(10):8508-15. doi: 10.1074/jbc.M208231200. Epub 2002 Dec 20. J Biol Chem. 2003. PMID: 12496258
-
PAR-2 activation in intestinal epithelial cells potentiates interleukin-1beta-induced chemokine secretion via MAP kinase signaling pathways.Cytokine. 2005 Sep 7;31(5):358-67. doi: 10.1016/j.cyto.2005.06.004. Cytokine. 2005. PMID: 16095910
-
Proteinase-activated receptors induce interleukin-8 expression by intestinal epithelial cells through ERK/RSK90 activation and histone acetylation.FASEB J. 2010 Jun;24(6):1971-80. doi: 10.1096/fj.09-137646. Epub 2010 Jan 11. FASEB J. 2010. PMID: 20065107
-
[Characterization of endothelial progenitor cells and putative strategies to improve their expansion].J Soc Biol. 2009;203(2):197-207. doi: 10.1051/jbio/2009024. Epub 2009 Jun 16. J Soc Biol. 2009. PMID: 19527634 Review. French.
Cited by
-
Expression of protease-activated receptor 1 and 2 and anti-tubulogenic activity of protease-activated receptor 1 in human endothelial colony-forming cells.PLoS One. 2014 Oct 7;9(10):e109375. doi: 10.1371/journal.pone.0109375. eCollection 2014. PLoS One. 2014. PMID: 25289673 Free PMC article.
-
Endothelial progenitor cells in ischemic stroke: an exploration from hypothesis to therapy.J Hematol Oncol. 2015 Apr 11;8:33. doi: 10.1186/s13045-015-0130-8. J Hematol Oncol. 2015. PMID: 25888494 Free PMC article. Review.
-
Transient extremity ischemia augments CD34+ progenitor cell availability.Stem Cell Rev Rep. 2011 Sep;7(3):639-45. doi: 10.1007/s12015-011-9234-x. Stem Cell Rev Rep. 2011. PMID: 21318598
-
Brain endothelial cells synthesize neurotoxic thrombin in Alzheimer's disease.Am J Pathol. 2010 Apr;176(4):1600-6. doi: 10.2353/ajpath.2010.090406. Epub 2010 Feb 11. Am J Pathol. 2010. PMID: 20150433 Free PMC article.
-
Endothelial progenitor cells in the host defense response.Pharmacol Ther. 2023 Jan;241:108315. doi: 10.1016/j.pharmthera.2022.108315. Epub 2022 Nov 24. Pharmacol Ther. 2023. PMID: 36436689 Free PMC article. Review.
References
-
- Murphy PM, Baggiolini M, Charo IF, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000; 1: 145–76. - PubMed
-
- Li A, Dubey S, Varney ML, et al. IL‐8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003; 6: 3369–76. - PubMed
-
- Galffy G, Mohammed KA, Nasreen N, et al. Inhibition of interleukin‐8 reduces human malignant pleural mesothelioma propagation in nude mouse model. Oncol Res. 1999; 4: 187–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

