Tic40 is important for reinsertion of proteins from the chloroplast stroma into the inner membrane
- PMID: 18657235
- PMCID: PMC2667645
- DOI: 10.1111/j.1365-313X.2008.03638.x
Tic40 is important for reinsertion of proteins from the chloroplast stroma into the inner membrane
Abstract
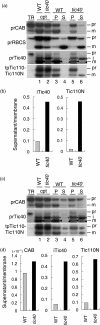
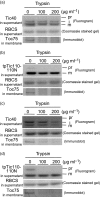
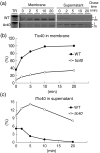
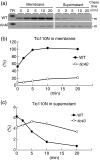
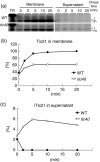
Chloroplast inner-membrane proteins Tic40 and Tic110 are first imported from the cytosol into the chloroplast stroma, and subsequently reinserted from the stroma into the inner membrane. However, the mechanism of reinsertion remains unclear. Here we show that Tic40 itself is involved in this reinsertion process. When precursors of either Tic40 or a Tic110 C-terminal truncate, tpTic110-Tic110N, were imported into chloroplasts isolated from a tic40-null mutant, soluble Tic40 and Tic110N intermediates accumulated in the stroma of tic40-mutant chloroplasts, due to a slower rate of reinsertion. We further show that a larger quantity of soluble Tic21 intermediates also accumulated in the stroma of tic40-mutant chloroplasts. In contrast, inner-membrane insertion of the triose-phosphate/phosphate translocator was not affected by the tic40 mutation. Our data suggest that multiple pathways exist for the insertion of chloroplast inner-membrane proteins.
Figures


References
-
- Bedard J, Kubis S, Bimanadham S, Jarvis P. Functional similarity between the chloroplast translocon component, Tic40, and the human co-chaperone, Hsp70-interacting protein (Hip) J. Biol. Chem. 2007;282:21404–21414. - PubMed
-
- Brink S, Fischer K, Klosgen R-B, Flugge U-I. Sorting of nuclear-encoded chloroplast membrane proteins to the envelope and the thylakoid membrane. J. Biol. Chem. 1995;270:20808–20815. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

