Paclitaxel loading in PLGA nanospheres affected the in vitro drug cell accumulation and antiproliferative activity
- PMID: 18657273
- PMCID: PMC2519087
- DOI: 10.1186/1471-2407-8-212
Paclitaxel loading in PLGA nanospheres affected the in vitro drug cell accumulation and antiproliferative activity
Abstract
Background: PTX is one of the most widely used drug in oncology due to its high efficacy against solid tumors and several hematological cancers. PTX is administered in a formulation containing 1:1 Cremophor EL (polyethoxylated castor oil) and ethanol, often responsible for toxic effects. Its encapsulation in colloidal delivery systems would gain an improved targeting to cancer cells, reducing the dose and frequency of administration.
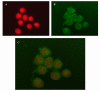
Methods: In this paper PTX was loaded in PLGA NS. The activity of PTX-NS was assessed in vitro against thyroid, breast and bladder cancer cell lines in cultures. Cell growth was evaluated by MTS assay, intracellular NS uptake was performed using coumarin-6 labelled NS and the amount of intracellular PTX was measured by HPLC.
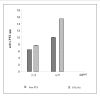
Results: NS loaded with 3% PTX (w/w) had a mean size < 250 nm and a polydispersity index of 0.4 after freeze-drying with 0.5% HP-Cyd as cryoprotector. PTX encapsulation efficiency was 30% and NS showed a prolonged drug release in vitro. An increase of the cytotoxic effect of PTX-NS was observed with respect to free PTX in all cell lines tested.
Conclusion: These findings suggest that the greater biological effect of PTX-NS could be due to higher uptake of the drug inside the cells as shown by intracellular NS uptake and cell accumulation studies.
Figures





Similar articles
-
Enhanced in vitro antiproliferative effects of EpCAM antibody-functionalized paclitaxel-loaded PLGA nanoparticles in retinoblastoma cells.Mol Vis. 2011;17:2724-37. Epub 2011 Oct 19. Mol Vis. 2011. Retraction in: Mol Vis. 2013 Jun 06;19:1258. PMID: 22065926 Free PMC article. Retracted.
-
Development of PLGA-based itraconazole injectable nanospheres for sustained release.Int J Nanomedicine. 2013;8:4521-31. doi: 10.2147/IJN.S54040. Epub 2013 Nov 21. Int J Nanomedicine. 2013. PMID: 24311942 Free PMC article.
-
Development of innovative paclitaxel-loaded small PLGA nanoparticles: study of their antiproliferative activity and their molecular interactions on prostatic cancer cells.Int J Pharm. 2013 Oct 1;454(2):712-9. doi: 10.1016/j.ijpharm.2013.05.018. Epub 2013 May 21. Int J Pharm. 2013. PMID: 23707251
-
Paclitaxel-loaded PEGylated PLGA-based nanoparticles: in vitro and in vivo evaluation.J Control Release. 2009 Jan 5;133(1):11-7. doi: 10.1016/j.jconrel.2008.09.086. Epub 2008 Oct 9. J Control Release. 2009. PMID: 18950666
-
Enabling anticancer therapeutics by nanoparticle carriers: the delivery of Paclitaxel.Int J Mol Sci. 2011;12(7):4395-413. doi: 10.3390/ijms12074395. Epub 2011 Jul 7. Int J Mol Sci. 2011. PMID: 21845085 Free PMC article. Review.
Cited by
-
Encapsulation of alpha-1 antitrypsin in PLGA nanoparticles: in vitro characterization as an effective aerosol formulation in pulmonary diseases.J Nanobiotechnology. 2012 May 20;10:20. doi: 10.1186/1477-3155-10-20. J Nanobiotechnology. 2012. PMID: 22607686 Free PMC article.
-
Antiproliferative Effect of Methanolic Extract of Vernonia greggii (Asteraceae) on Human Tumoral HeLa Cells Nanoencapsulated into PLGA-Nanoparticles.Materials (Basel). 2025 Jan 27;18(3):580. doi: 10.3390/ma18030580. Materials (Basel). 2025. PMID: 39942246 Free PMC article.
-
Aptamer-labeled PLGA nanoparticles for targeting cancer cells.Cancer Nanotechnol. 2012;3(1-6):1-12. doi: 10.1007/s12645-011-0024-6. Epub 2012 Jan 19. Cancer Nanotechnol. 2012. PMID: 26069492 Free PMC article.
-
Novel Nano-Therapeutic Approach Actively Targets Human Ovarian Cancer Stem Cells after Xenograft into Nude Mice.Int J Mol Sci. 2017 Apr 12;18(4):813. doi: 10.3390/ijms18040813. Int J Mol Sci. 2017. PMID: 28417924 Free PMC article.
-
Midkine as a potential diagnostic marker in epithelial ovarian cancer for cisplatin/paclitaxel combination clinical therapy.Am J Cancer Res. 2015 Jan 15;5(2):629-38. eCollection 2015. Am J Cancer Res. 2015. PMID: 25973302 Free PMC article.
References
-
- Ain KB, Egorin MJ, De Simone PA. Treatment of anaplastic thyroid carcinoma with paclitaxel: phase 2 trial using ninety-six-hour infusion. Collaborative Anaplastic Thyroid Cancer Health Intervention Trials (CATCHIT) Group. Thyroid. 2000;10:587–94. - PubMed
-
- Chougule PB, Akhtar MS, Rathore R, Koness J, McRae R, Nigri P, Radie-Keane K, Kennedy T, Wanebo HJ, Ready N. Concurrent chemoradiotherapy with weekly paclitaxel and carboplatin for locally advanced head and neck cancer: Long-term follow-up of a brown university oncology group phase II study (HN-53) Head Neck. 2008;30:289–296. doi: 10.1002/hed.20700. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

