Psychological stress impairs the local CD8+ T cell response to mucosal HSV-1 infection and allows for increased pathogenicity via a glucocorticoid receptor-mediated mechanism
- PMID: 18657369
- PMCID: PMC3721759
- DOI: 10.1016/j.psyneuen.2008.04.010
Psychological stress impairs the local CD8+ T cell response to mucosal HSV-1 infection and allows for increased pathogenicity via a glucocorticoid receptor-mediated mechanism
Abstract
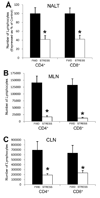
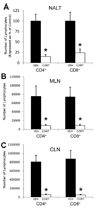
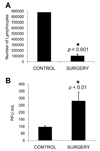
Psychological stress and its associated increases in corticosterone are generally immunosuppressive and contribute to increased herpes simplex virus (HSV)-associated pathogenicity. However, the impact of stress on local control of the initial mucosal-based HSV infection has not been elucidated, nor have the ramifications of such failures of the immune response in terms of viral spread. To address these gaps in knowledge, the studies described herein sought to determine how psychological stress and associated increases in corticosterone may increase susceptibility to HSV encephalitis by allowing for increased viral titers at the site of initial infection. We have shown that in mice intranasally infected with HSV-1, a cell-mediated immune response occurs in the nasopharyngeal-associated lymphoid tissue (NALT), mediastinal lymph nodes (MLN), and superficial cervical lymph nodes (CLN). However, psychological stress induced by restraint decreased the number of lymphocytes in these tissues in HSV-infected mice. Surprisingly, the effects of this restraint stress on HSV-specific CTL function varied by immune tissue. Increased viral titers were found in the nasal cavity of stressed mice, an observation which correlated with an increased CD8+ cell response in the CLN. These findings led us to extend our studies to also determine the ramifications of decreased numbers of locally derived lymphocytes on viral titers following infection. Using an approach in which the NALT was surgically removed prior to infection, we confirmed that decreased numbers of NALT-derived lymphocytes at the time of infection allows for increased viral replication. We conclude that the increased viral titers observed in mice experiencing psychological stress are the consequence of a glucocorticoid-mediated reduction in the numbers of lymphocytes responsible for resolving the initial infection.
Figures



References
-
- Akagi T, Ueno M, Hiraishi K, Baba M, Akashi M. AIDS vaccine: Intranasal immunization using inactivated HIV-1-capturing core-corona type polymeric nanospheres. J Control Release. 2005;109:49–61. - PubMed
-
- Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. - PubMed
-
- Anglen CS, Truckenmiller ME, Schell TD, Bonneau RH. The dual role of CD8+ T lymphocytes in the development of stress-induced herpes simplex encephalitis. J Neuroimmunol. 2003;140:13–27. - PubMed
-
- Asanuma H, Thompson AH, Iwasaki T, Sato Y, Inaba Y, Aizawa C, Kurata T, Tamura S. Isolation and characterization of mouse nasal-associated lymphoid tissue. J Immunol Methods. 1997;202:123–131. - PubMed
-
- Bailey M, Engler H, Hunzeker J, Sheridan JF. The hypothalamic-pituitary-adrenal axis and viral infection. Viral Immunol. 2003;16:141–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

