The effect of fixed charge modifications on electron capture dissociation
- PMID: 18657441
- PMCID: PMC3116146
- DOI: 10.1016/j.jasms.2008.06.014
The effect of fixed charge modifications on electron capture dissociation
Abstract
Electron capture dissociation (ECD) studies of two modified amyloid beta peptides (20-29 and 25-35) were performed to investigate the role of H* radicals in the ECD of peptide ions and the free-radical cascade (FRC) mechanism. 2,4,6-Trimethylpyridinium (TMP) was used as the fixed charge tag, which is postulated to both trap the originally formed radical upon electron capture and inhibit the H* generation. It was found that both the number and locations of the fixed charge groups influenced the backbone and side-chain cleavages of these peptides in ECD. In general, the frequency and extent of backbone cleavages decreased and those of side-chain cleavages increased with the addition of fixed charge tags. A singly labeled peptide with the tag group farther away from the protonated site experienced a smaller abundance decrease in backbone cleavage fragments than the one with the tag group closer to the protonated site. Despite the nonprotonated nature of all charge carriers in doubly labeled peptide ions, several c and z* ions were still observed in their ECD spectra. Thus, although H* transfer may be important for the NC(alpha) bond cleavage, there also exist other pathways, which would require a radical migration via H* abstraction through space or via an amide superbase mechanism. Finally, internal fragment ions were observed in the ECD of these linear peptides, indicating that the important role of the FRC in backbone cleavages is not limited to the ECD of cyclic peptides.
Figures


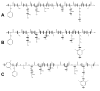

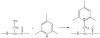


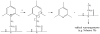
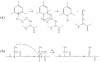
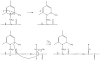
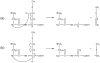
References
-
- Little DP, Speir JP, Senko MW, Oconnor PB, McLafferty FW. Infrared Multiphoton Dissociation of Large Multiply-Charged Ions for Biomolecule Sequencing. Anal Chem. 1994;66:2809–2815. - PubMed
-
- Gauthier JW, Trautman TR, Jacobson DB. Sustained Off-Resonance Irradiation for Collision-Activated Dissociation Involving Fourier-Transform Mass-Spectrometry - Collision-Activated Dissociation Technique That Emulates Infrared Multiphoton Dissociation. Anal Chim Acta. 1991;246:211–225.
-
- Zubarev RA, Kelleher NL, McLafferty FW. Electron capture dissociation of multiply charged protein cations. A nonergodic process. J Am Chem Soc. 1998;120:3265–3266.
-
- Kelleher RL, Zubarev RA, Bush K, Furie B, Furie BC, McLafferty FW, Walsh CT. Localization of labile posttranslational modifications by electron capture dissociation: The case of gamma-carboxyglutamic acid. Anal Chem. 1999;71:4250–4253. - PubMed
-
- Stensballe A, Jensen ON, Olsen JV, Haselmann KF, Zubarev RA. Electron capture dissociation of singly and multiply phosphorylated peptides. Rapid Commun Mass Spectrom. 2000;14:1793–1800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

