Acetylcholinesterase: mechanisms of covalent inhibition of H447I mutant determined by computational analyses
- PMID: 18657802
- PMCID: PMC2576475
- DOI: 10.1016/j.cbi.2008.04.044
Acetylcholinesterase: mechanisms of covalent inhibition of H447I mutant determined by computational analyses
Abstract
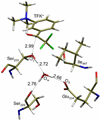
The reaction mechanisms of two inhibitor TFK(+) and TFK(0) binding to H447I mutant mouse acetylcholinesterase (mAChE) have been investigated by using a combined ab initio quantum mechanical/molecular mechanical (QM/MM) approach and classical molecular dynamics (MD) simulations. TFK(+) binding to the H447I mutant may proceed with a different reaction mechanism from the wild-type. A water molecule takes over the role of His447 and participates in the bond breaking and forming as a "charge relayer". Unlike in the wild-type mAChE case, Glu334, a conserved residue from the catalytic triad, acts as a catalytic base in the reaction. The calculated energy barrier for this reaction is about 8kcal/mol. These predictions await experimental verification. In the case of the neutral ligand TFK(0), however, multiple MD simulations on the TFK(0)/H447I complex reveal that none of the water molecules can be retained in the active site as a "catalytic" water. Taken together our computational studies confirm that TFK(0) is almost inactive in the H447I mutant, and also provide detailed mechanistic insights into the experimental observations.
Figures


References
-
- Rosenberry TL. Acetylcholinesterase. Adv. Enzymol. Relat. Areas Mol. Biol. 1975;43:103–218. - PubMed
-
- Schumacher M, Camp S, Maulet Y, Newton M, Macpheequigley K, Taylor SS, Friedmann T, Taylor P. Primary structure of acetylcholinesterase - implications for regulation and function. Fed. Proc. 1986;45:2976–2981. - PubMed
-
- Taylor P. The Pharmacological Basis of Therapeutics. New York: MacMillan; 1985.
-
- Contestabile A, Fila T, Bartesaghi R, Contestabile A, Ciani E. Choline acetyltransferase activity at different ages in brain of ts65dn mice, an animal model for down's syndrome and related neurodegenerative diseases. J. Neurochem. 2006;97:515–526. - PubMed
-
- Piazzi L, Rampa A, Bisi A, Gobbi S, Belluti F, Cavalli A, Bartolini M, Andrisano V, Valenti P, Recanatini M. 3-(4-{[benzyl(methyl)amino]methyl}-phenyl)-6,7-dimethoxy-2h-2-chromenone (ap2238) inhibits both acetylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation: a dual function lead for alzheimer's disease therapy. J. Med. Chem. 2003;46:2279–2282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

