The LIM protein LIMD1 influences osteoblast differentiation and function
- PMID: 18657804
- PMCID: PMC2570157
- DOI: 10.1016/j.yexcr.2008.06.003
The LIM protein LIMD1 influences osteoblast differentiation and function
Abstract
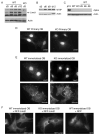
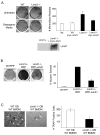
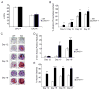
The balance between bone resorption and bone formation involves the coordinated activities of osteoblasts and osteoclasts. Communication between these two cell types is essential for maintenance of normal bone homeostasis; however, the mechanisms regulating this cross talk are not completely understood. Many factors that mediate differentiation and function of both osteoblasts and osteoclasts have been identified. The LIM protein Limd1 has been implicated in the regulation of stress osteoclastogenesis through an interaction with the p62/sequestosome protein. Here we show that Limd1 also influences osteoblast progenitor numbers, differentiation, and function. Limd1(-/-) calvarial osteoblasts display increased mineralization and accelerated differentiation. While no significant differences in osteoblast number or function were detected in vivo, bone marrow stromal cells isolated from Limd1(-/-) mice contain significantly more osteoblast progenitors compared to wild type controls when cultured ex vivo. Furthermore, we observed a significant increase in nuclear beta-catenin staining in differentiating Limd1(-/-) calvarial osteoblasts suggesting that Limd1 is a negative regulator of canonical Wnt signaling in osteoblasts. These results demonstrate that Limd1 influences not only stress osteoclastogenesis but also osteoblast function and osteoblast progenitor commitment. Together, these data identify Limd1 as a novel regulator of both bone osetoclast and bone osteoblast development and function.
Figures



References
-
- Roodman GD. Regulation of osteoclast differentiation. Ann N Y Acad Sci. 2006;1068:100–9. - PubMed
-
- Rogers A, Eastell R. Circulating osteoprotegerin and receptor activator for nuclear factor kappaB ligand: clinical utility in metabolic bone disease assessment. J Clin Endocrinol Metab. 2005;90:6323–31. - PubMed
-
- Kiss H, Kedra D, Yang Y, Kost-Alimova M, Kiss C, O’Brien KP, Fransson I, Klein G, Imreh S, Dumanski JP. A novel gene containing LIM domains (LIMD1) is located within the common eliminated region 1 (C3CER1) in 3p21.3. Hum Genet. 1999;105:552–9. - PubMed
-
- Crawford AW, Beckerle MC. Purification and characterization of zyxin, an 82,000-dalton component of adherens junctions. J Biol Chem. 1991;266:5847–53. - PubMed
-
- Petit MM, Mols R, Schoenmakers EF, Mandahl N, Van de Ven WJ. LPP, the preferred fusion partner gene of HMGIC in lipomas, is a novel member of the LIM protein gene family. Genomics. 1996;36:118–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

