Hypoxic vasodilation by red blood cells: evidence for an s-nitrosothiol-based signal
- PMID: 18658051
- PMCID: PMC2763414
- DOI: 10.1161/CIRCRESAHA.108.176867
Hypoxic vasodilation by red blood cells: evidence for an s-nitrosothiol-based signal
Abstract
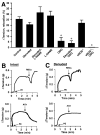
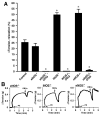
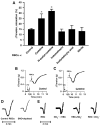
Red blood cells (RBCs) have been ascribed an essential role in matching blood flow to local metabolic demand during hypoxic vasodilation. The vasodilatory function of RBCs evidently relies on the allosteric properties of hemoglobin (Hb), which couple the conformation of Hb to tissue oxygen tension (Po(2)) and thereby provide a basis for the graded vasodilatory activity that is inversely proportional to Hb oxygen saturation. Although a large body of evidence indicates that the Po(2)-coupled allosteric transition from R (oxy)-state to T (deoxy)-state subserves the release from Hb of vasodilatory nitric oxide (NO) bioactivity, it has not yet been determined whether the NO-based signal is a necessary and sufficient source of RBC-mediated vasoactivity and it has been suggested that ATP or nitrite may also contribute. We demonstrate here by bioassay that untreated human RBCs rapidly and substantially relax thoracic aorta from both rabbit and mouse at low Po(2) (approximately 1% O(2)) but not at high Po(2) (approximately 21% O(2)). RBC-mediated vasorelaxation is inhibited by prior depletion of S-nitroso-Hb, potentiated by low-molecular-weight thiols, and dependent on cGMP. Furthermore, these relaxations are largely endothelium-independent and unaffected by NO synthase inhibition or nitrite. Robust relaxations by RBCs are also elicited in the absence of endothelial, neuronal or inducible NO synthase. Finally, contractions that appear following resolution of RBC-mediated relaxations are dependent on NO derived from RBCs as well as the endothelium. Our results suggest that an S-nitrosothiol-based signal originating from RBCs mediates hypoxic vasodilation by RBCs, and that vasorelaxation by RBCs dominates NO-based vasoconstriction under hypoxic conditions.
Figures


Comment in
-
Transnitrosation signals oxyhemoglobin desaturation.Circ Res. 2008 Aug 29;103(5):441-3. doi: 10.1161/CIRCRESAHA.108.183442. Circ Res. 2008. PMID: 18757830 Free PMC article. No abstract available.
Similar articles
-
Optimized S-nitrosohemoglobin Synthesis in Red Blood Cells to Preserve Hypoxic Vasodilation Via βCys93.J Pharmacol Exp Ther. 2022 Jul;382(1):1-10. doi: 10.1124/jpet.122.001194. Epub 2022 May 5. J Pharmacol Exp Ther. 2022. PMID: 35512801 Free PMC article.
-
Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology: Developments on a Three-Gas Respiratory Cycle.Circ Res. 2020 Jan 3;126(1):129-158. doi: 10.1161/CIRCRESAHA.119.315626. Epub 2019 Oct 8. Circ Res. 2020. PMID: 31590598 Free PMC article. Review.
-
Nitrite enhances RBC hypoxic ATP synthesis and the release of ATP into the vasculature: a new mechanism for nitrite-induced vasodilation.Am J Physiol Heart Circ Physiol. 2009 Oct;297(4):H1494-503. doi: 10.1152/ajpheart.01233.2008. Epub 2009 Aug 21. Am J Physiol Heart Circ Physiol. 2009. PMID: 19700624 Free PMC article.
-
Pulmonary vascular effects of red blood cells containing S-nitrosated hemoglobin.Am J Physiol Heart Circ Physiol. 2004 Dec;287(6):H2561-8. doi: 10.1152/ajpheart.00310.2004. Epub 2004 Aug 5. Am J Physiol Heart Circ Physiol. 2004. PMID: 15297254
-
The dual roles of red blood cells in tissue oxygen delivery: oxygen carriers and regulators of local blood flow.J Exp Biol. 2009 Nov;212(Pt 21):3387-93. doi: 10.1242/jeb.023697. J Exp Biol. 2009. PMID: 19837879 Review.
Cited by
-
"Beet-ing" the Mountain: A Review of the Physiological and Performance Effects of Dietary Nitrate Supplementation at Simulated and Terrestrial Altitude.Sports Med. 2017 Nov;47(11):2155-2169. doi: 10.1007/s40279-017-0744-9. Sports Med. 2017. PMID: 28577258 Free PMC article. Review.
-
Red cell physiology and signaling relevant to the critical care setting.Curr Opin Pediatr. 2015 Jun;27(3):267-76. doi: 10.1097/MOP.0000000000000225. Curr Opin Pediatr. 2015. PMID: 25888155 Free PMC article. Review.
-
Nitric oxide signaling in the microcirculation.Crit Rev Biomed Eng. 2011;39(5):397-433. doi: 10.1615/critrevbiomedeng.v39.i5.40. Crit Rev Biomed Eng. 2011. PMID: 22196161 Free PMC article. Review.
-
Effects of maternal hypoxia on muscle vasodilatation evoked by acute systemic hypoxia in adult rat offspring: changed roles of adenosine and A1 receptors.J Physiol. 2010 Dec 15;588(Pt 24):5115-25. doi: 10.1113/jphysiol.2010.198275. Epub 2010 Oct 20. J Physiol. 2010. PMID: 20962006 Free PMC article.
-
Optimized S-nitrosohemoglobin Synthesis in Red Blood Cells to Preserve Hypoxic Vasodilation Via βCys93.J Pharmacol Exp Ther. 2022 Jul;382(1):1-10. doi: 10.1124/jpet.122.001194. Epub 2022 May 5. J Pharmacol Exp Ther. 2022. PMID: 35512801 Free PMC article.
References
-
- Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. - PubMed
-
- Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409:622–626. - PubMed
-
- Allen BW, Piantadosi CA. How do red blood cells cause hypoxic vasodilation? The SNO-hemoglobin paradigm. Am J Physiol Heart Circ Physiol. 2006;291:H1507–H1512. - PubMed
-
- McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, Piantadosi CA, Stamler JS. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

