Functional rescue of degenerating photoreceptors in mice homozygous for a hypomorphic cGMP phosphodiesterase 6 b allele (Pde6bH620Q)
- PMID: 18658088
- PMCID: PMC2715364
- DOI: 10.1167/iovs.07-1422
Functional rescue of degenerating photoreceptors in mice homozygous for a hypomorphic cGMP phosphodiesterase 6 b allele (Pde6bH620Q)
Abstract
Purpose: Approximately 8% of autosomal recessive retinitis pigmentosa (RP) cases worldwide are due to defects in rod-specific phosphodiesterase PDE6, a tetramer consisting of catalytic (PDE6alpha and PDE6beta) and two regulatory (PDE6gamma) subunits. In mice homozygous for a nonsense Pde6b(rd1) allele, absence of PDE6 activity is associated with retinal disease similar to humans. Although studied for 80 years, the rapid degeneration Pde6b(rd1) phenotype has limited analyses and therapeutic modeling. Moreover, this model does not represent human RP involving PDE6B missense mutations. In the current study the mouse missense allele, Pde6b(H620Q) was characterized further.
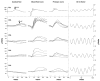
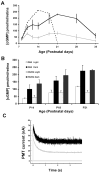
Methods: Photoreceptor degeneration in Pde6b(H620Q) homozygotes was documented by histochemistry, whereas PDE6beta expression and activity were monitored by immunoblotting and cGMP assays. To measure changes in rod physiology, electroretinograms and intracellular Ca(2+) recording were performed. To test the effectiveness of gene therapy, Opsin::Pde6b lentivirus was subretinally injected into Pde6b(H620Q) homozygotes.
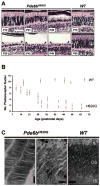
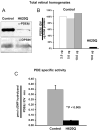
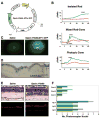
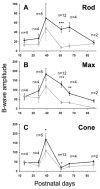
Results: Within 3 weeks of birth, the Pde6b(H620Q) homozygotes displayed relatively normal photoreceptors, but by 7 weeks degeneration was largely complete. Before degeneration, PDE6beta expression and PDE6 activity were reduced. Although light-/dark-adapted total cGMP levels appeared normal, Pde6b(H620Q) homozygotes exhibited depressed rod function and elevated outer segment Ca(2+). Transduction with Opsin::Pde6b lentivirus resulted in histologic and functional rescue of photoreceptors.
Conclusions: Pde6b(H620Q) homozygous mice exhibit a hypomorphic phenotype with partial PDE6 activity that may result in an increased Ca(2+) to promote photoreceptor death. As degeneration in Pde6b(H620Q) mutants is slower than in Pde6b(rd1) mice and can be suppressed by Pde6b transduction, this Pde6b(H620Q) model may provide an alternate means to explore new treatments of RP.
Figures
References
-
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119 –1122. - PubMed
-
- Klein R, Klein BE, Jensen SC, Mares-Perlman JA, Cruickshanks KJ, Palta M. Age-related maculopathy in a multiracial United States population: the National Health and Nutrition Examination Survey III. Ophthalmology. 1999;106:1056 –1065. - PubMed
-
- Klein R, Rowland ML, Harris MI. Racial/ethnic differences in age-related maculopathy. Third National Health and Nutrition Examination Survey. Ophthalmology. 1995;102:371–381. - PubMed
-
- Hahn LB, Berson EL, Dryja TP. Evaluation of the gene encoding the gamma subunit of rod phosphodiesterase in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1994;35:1077–1082. - PubMed
-
- Huang SH, Pittler SJ, Huang X, Oliveira L, Berson EL, Dryja TP. Autosomal recessive retinitis pigmentosa caused by mutations in the alpha subunit of rod cGMP phosphodiesterase. Nat Genet. 1995;11:468 – 471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

