Increased dosage of Dyrk1A alters alternative splicing factor (ASF)-regulated alternative splicing of tau in Down syndrome
- PMID: 18658135
- PMCID: PMC2568927
- DOI: 10.1074/jbc.M802645200
Increased dosage of Dyrk1A alters alternative splicing factor (ASF)-regulated alternative splicing of tau in Down syndrome
Abstract
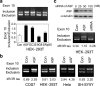
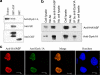
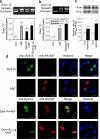
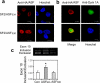
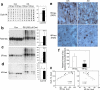
Two groups of tau, 3R- and 4R-tau, are generated by alternative splicing of tau exon 10. Normal adult human brain expresses equal levels of them. Disruption of the physiological balance is a common feature of several tauopathies. Very early in their life, individuals with Down syndrome (DS) develop Alzheimer-type tau pathology, the molecular basis for which is not fully understood. Here, we demonstrate that Dyrk1A, a kinase encoded by a gene in the DS critical region, phosphorylates alternative splicing factor (ASF) at Ser-227, Ser-234, and Ser-238, driving it into nuclear speckles and preventing it from facilitating tau exon 10 inclusion. The increased dosage of Dyrk1A in DS brain due to trisomy of chromosome 21 correlates to an increase in 3R-tau level, which on abnormal hyperphosphorylation and aggregation of tau results in neurofibrillary degeneration. Imbalance of 3R- and 4R-tau in DS brain by Dyrk1A-induced dysregulation of alternative splicing factor-mediated alternative splicing of tau exon 10 represents a novel mechanism of neurofibrillary degeneration and may help explain early onset tauopathy in individuals with DS.
Figures


References
-
- Grundke-Iqbal, I., Iqbal, K., Quinlan, M., Tung, Y. C., Zaidi, M. S., and Wisniewski, H. M. (1986) J. Biol. Chem. 261 6084-6089 - PubMed
-
- Ballatore, C., Lee, V. M., and Trojanowski, J. Q. (2007) Nat. Rev. Neurosci. 8 663-672 - PubMed
-
- Iqbal, K., Alonso, A. C., Chen, S., Chohan, M. O., El-Akkad, E., Gong. C.-X., Khatoon, S., Li, B., Liu, F., Rahman, A., Tanimukai, H., and Grundke-Iqbal, I. (2005) Biochim. Biophys. Acta 1739 198-210 - PubMed
-
- Goedert, M., Spillantini, M. G., Jakes, R., Rutherford, D., and Crowther, R. A. (1989) Neuron 3 519-526 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

