Rapid-reaction kinetic characterization of the pathway of streptokinase-plasmin catalytic complex formation
- PMID: 18658146
- PMCID: PMC2533780
- DOI: 10.1074/jbc.M804038200
Rapid-reaction kinetic characterization of the pathway of streptokinase-plasmin catalytic complex formation
Abstract
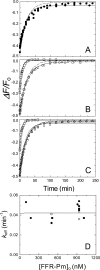
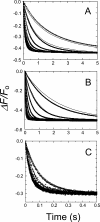
Binding of the fibrinolytic proteinase plasmin (Pm) to streptokinase (SK) in a tight stoichiometric complex transforms Pm into a potent proteolytic activator of plasminogen. SK binding to the catalytic domain of Pm, with a dissociation constant of 12 pm, is assisted by SK Lys(414) binding to a Pm kringle, which accounts for a 11-20-fold affinity decrease when Pm lysine binding sites are blocked by 6-aminohexanoic acid (6-AHA) or benzamidine. The pathway of SK.Pm catalytic complex formation was characterized by stopped-flow kinetics of SK and the Lys(414) deletion mutant (SKDeltaK414) binding to Pm labeled at the active site with 5-fluorescein ([5F]FFR-Pm) and the reverse reactions by competitive displacement of [5F]FFR-Pm with active site-blocked Pm. The rate constants for the biexponential fluorescence quenching caused by SK and SKDeltaK414 binding to [5F]FFR-Pm were saturable as a function of SK concentration, reporting encounter complex affinities of 62-110 nm in the absence of lysine analogs and 4900-6500 and 1430-2200 nm in the presence of 6-AHA and benzamidine, respectively. The encounter complex with SKDeltaK414 was approximately 10-fold weaker in the absence of lysine analogs but indistinguishable from that of native SK in the presence of 6-AHA and benzamidine. The studies delineate for the first time the sequence of molecular events in the formation of the SK.Pm catalytic complex and its regulation by kringle ligands. Analysis of the forward and reverse reactions supports a binding mechanism in which SK Lys(414) binding to a Pm kringle accompanies near-diffusion-limited encounter complex formation followed by two slower, tightening conformational changes.
Figures




Similar articles
-
Rapid binding of plasminogen to streptokinase in a catalytic complex reveals a three-step mechanism.J Biol Chem. 2014 Oct 3;289(40):28006-18. doi: 10.1074/jbc.M114.589077. Epub 2014 Aug 19. J Biol Chem. 2014. PMID: 25138220 Free PMC article.
-
Role of the streptokinase alpha-domain in the interactions of streptokinase with plasminogen and plasmin.J Biol Chem. 2005 Mar 4;280(9):7504-10. doi: 10.1074/jbc.M411637200. Epub 2004 Dec 28. J Biol Chem. 2005. PMID: 15623524 Free PMC article.
-
Binding of the COOH-terminal lysine residue of streptokinase to plasmin(ogen) kringles enhances formation of the streptokinase.plasmin(ogen) catalytic complexes.J Biol Chem. 2006 Sep 15;281(37):26774-8. doi: 10.1074/jbc.C600171200. Epub 2006 Jul 20. J Biol Chem. 2006. PMID: 16857686 Free PMC article.
-
Plasminogen substrate recognition by the streptokinase-plasminogen catalytic complex is facilitated by Arg253, Lys256, and Lys257 in the streptokinase beta-domain and kringle 5 of the substrate.J Biol Chem. 2009 Jul 17;284(29):19511-21. doi: 10.1074/jbc.M109.005512. Epub 2009 May 27. J Biol Chem. 2009. PMID: 19473980 Free PMC article.
-
Streptokinase binds preferentially to the extended conformation of plasminogen through lysine binding site and catalytic domain interactions.Biochemistry. 2000 Nov 14;39(45):13974-81. doi: 10.1021/bi000594i. Biochemistry. 2000. PMID: 11076540
Cited by
-
Fluorescent reporters of thrombin, heparin cofactor II, and heparin binding in a ternary complex.Anal Biochem. 2012 Feb 15;421(2):489-98. doi: 10.1016/j.ab.2011.11.021. Epub 2011 Dec 6. Anal Biochem. 2012. PMID: 22206940 Free PMC article.
-
Rapid binding of plasminogen to streptokinase in a catalytic complex reveals a three-step mechanism.J Biol Chem. 2014 Oct 3;289(40):28006-18. doi: 10.1074/jbc.M114.589077. Epub 2014 Aug 19. J Biol Chem. 2014. PMID: 25138220 Free PMC article.
-
Structural Biology and Protein Engineering of Thrombolytics.Comput Struct Biotechnol J. 2019 Jul 2;17:917-938. doi: 10.1016/j.csbj.2019.06.023. eCollection 2019. Comput Struct Biotechnol J. 2019. PMID: 31360331 Free PMC article. Review.
-
Pathogen activators of plasminogen.J Thromb Haemost. 2015 Jun;13 Suppl 1(0 1):S106-14. doi: 10.1111/jth.12939. J Thromb Haemost. 2015. PMID: 26149011 Free PMC article. Review.
-
Analytical methods for kinetic studies of biological interactions: A review.J Pharm Biomed Anal. 2015 Sep 10;113:163-80. doi: 10.1016/j.jpba.2015.01.042. Epub 2015 Jan 27. J Pharm Biomed Anal. 2015. PMID: 25700721 Free PMC article. Review.
References
-
- Boxrud, P. D., Verhamme, I. M., and Bock, P. E. (2004) J. Biol. Chem. 279 36633-36641 - PubMed
-
- Boxrud, P. D., and Bock, P. E. (2004) J. Biol. Chem. 279 36642-36649 - PubMed
-
- McClintock, D. K., and Bell, P. H. (1971) Biochem. Biophys. Res. Commun. 43 694-702 - PubMed
-
- Wohl, R. C., Summaria, L., Arzadon, L., and Robbins, K. C. (1978) J. Biol. Chem. 253 1402-1407 - PubMed
-
- Reddy, K. N., and Markus, G. (1972) J. Biol. Chem. 247 1683-1691 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

