External Ba2+ block of human Kv1.5 at neutral and acidic pH: evidence for Ho+-induced constriction of the outer pore mouth at rest
- PMID: 18658206
- PMCID: PMC2567935
- DOI: 10.1529/biophysj.108.133165
External Ba2+ block of human Kv1.5 at neutral and acidic pH: evidence for Ho+-induced constriction of the outer pore mouth at rest
Abstract
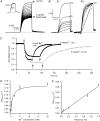
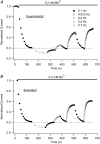
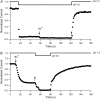
Previous studies have shown that low pHo accelerates depolarization-induced inactivation and decreases the macroscopic conductance by reducing channel availability. To test the hypothesis that outer pore constriction underlies the decreased conductance at low pHo, external Ba2+ was used to examine the accessibility of the channel pore at rest under neutral and acidic conditions. At pHo 7.4, Ba2+ block of closed channels follows a monoexponential time course and involves a low-affinity superficial site (KD congruent with 1 mM, -80 mV, 0 mM Ko(+)) and a high-affinity site (KD congruent with 4 microM) deeper in the pore. Depolarization promotes Ba2+ dissociation and an analytical model incorporating state-dependent changes of Ba2+ affinity is presented that replicates the frequency dependence of the time course and the extent of block. Open-channel block by Ba2+ is weak. At pHo 5.5, both the access to and exit from the deep site is inhibited. These results are consistent with a low-pHo-induced conformational change in the outer pore that prevents Ba2+ binding at rest or unbinding during depolarization. If a pore constriction is involved, similar to that proposed to occur during P/C-type inactivation, this would imply that closed-state inactivation in Kv1.5 occurs under acidic conditions.
Figures



Similar articles
-
Interaction of propofol with voltage-gated human Kv1.5 channel through specific amino acids within the pore region.Eur J Pharmacol. 2015 Oct 5;764:622-632. doi: 10.1016/j.ejphar.2015.08.007. Epub 2015 Aug 6. Eur J Pharmacol. 2015. PMID: 26256861
-
Localization of the K+ lock-In and the Ba2+ binding sites in a voltage-gated calcium-modulated channel. Implications for survival of K+ permeability.J Gen Physiol. 1999 Sep;114(3):365-76. doi: 10.1085/jgp.114.3.365. J Gen Physiol. 1999. PMID: 10469727 Free PMC article.
-
Separation of P/C- and U-type inactivation pathways in Kv1.5 potassium channels.J Physiol. 2005 Oct 1;568(Pt 1):31-46. doi: 10.1113/jphysiol.2005.087148. Epub 2005 Jul 14. J Physiol. 2005. PMID: 16020465 Free PMC article.
-
A novel type of internal barium block of a maxi-K+ channel from human vas deferens epithelial cells.Biophys J. 1998 Jan;74(1):199-209. doi: 10.1016/S0006-3495(98)77780-X. Biophys J. 1998. PMID: 9449323 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
-
Apoptosis of Corneal Epithelial Cells Caused by Ultraviolet B-induced Loss of K(+) is Inhibited by Ba(2.).Ocul Surf. 2016 Jul;14(3):401-9. doi: 10.1016/j.jtos.2016.05.001. Epub 2016 May 14. Ocul Surf. 2016. PMID: 27189864 Free PMC article.
-
Kinetic analysis of the effects of H+ or Ni2+ on Kv1.5 current shows that both ions enhance slow inactivation and induce resting inactivation.J Physiol. 2010 Aug 15;588(Pt 16):3011-30. doi: 10.1113/jphysiol.2010.191544. Epub 2010 Jun 25. J Physiol. 2010. PMID: 20581043 Free PMC article.
-
External Ba2+ block of the two-pore domain potassium channel TREK-1 defines conformational transition in its selectivity filter.J Biol Chem. 2011 Nov 18;286(46):39813-22. doi: 10.1074/jbc.M111.264788. Epub 2011 Sep 29. J Biol Chem. 2011. PMID: 21965685 Free PMC article.
-
Regulation of human cardiac Kv1.5 channels by extracellular acidification.Pflugers Arch. 2016 Nov;468(11-12):1885-1894. doi: 10.1007/s00424-016-1890-x. Epub 2016 Oct 28. Pflugers Arch. 2016. PMID: 27796577
-
ShakerIR and Kv1.5 mutant channels with enhanced slow inactivation also exhibit K⁺ o-dependent resting inactivation.Pflugers Arch. 2013 Nov;465(11):1545-55. doi: 10.1007/s00424-013-1297-x. Epub 2013 May 26. Pflugers Arch. 2013. PMID: 23708835
References
-
- Fedida, D., B. Wible, Z. Wang, B. Fermini, F. Faust, S. Nattel, and A. M. Brown. 1993. Identity of a novel delayed rectifier current from human heart with a cloned K+ channel current. Circ. Res. 73:210–216. - PubMed
-
- Feng, J., B. Wible, G. R. Li, Z. Wang, and S. Nattel. 1997. Antisense oligodeoxynucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier K+ current in cultured adult human atrial myocytes. Circ. Res. 80:572–579. - PubMed
-
- Fedida, D., S. Zhang, D. C. Kwan, C. Eduljee, and S. J. Kehl. 2005. Synergistic inhibition of the maximum conductance of Kv1.5 channels by extracellular K+ reduction and acidification. Cell Biochem. Biophys. 43:231–242. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

