Different types of cell-to-cell connections mediated by nanotubular structures
- PMID: 18658210
- PMCID: PMC2567924
- DOI: 10.1529/biophysj.108.131375
Different types of cell-to-cell connections mediated by nanotubular structures
Abstract
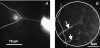
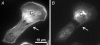
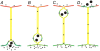
Communication between cells is crucial for proper functioning of multicellular organisms. The recently discovered membranous tubes, named tunneling nanotubes, that directly bridge neighboring cells may offer a very specific and effective way of intercellular communication. Our experiments on RT4 and T24 urothelial cell lines show that nanotubes that bridge neighboring cells can be divided into two types. The nanotubes of type I are shorter and more dynamic than those of type II, and they contain actin filaments. They are formed when cells explore their surroundings to make contact with another cell. The nanotubes of type II are longer and more stable than type I, and they have cytokeratin filaments. They are formed when two already connected cells start to move apart. On the nanotubes of both types, small vesicles were found as an integral part of the nanotubes (that is, dilatations of the nanotubes). The dilatations of type II nanotubes do not move along the nanotubes, whereas the nanotubes of type I frequently have dilatations (gondolas) that move along the nanotubes in both directions. A possible model of formation and mechanical stability of nanotubes that bridge two neighboring cells is discussed.
Figures











References
-
- Linder, M. E., and A. G. Gilman. 1992. G-proteins. Sci. Am. 267:36–43. - PubMed
-
- Kumar, N. M., and N. B. Gilula. 1996. The gap junction communication channel. Cell. 84:381–388. - PubMed
-
- Kralj-Iglič, V., G. Gomiscek, J. Majhenc, V. Arrigler, and S. Svetina. 2001. Myelin-like protrusions of giant phospholipid vesicles prepared by electroformation. Colloids Surf. A. 181:315–318.
-
- Kralj-Iglič, V., A. Iglič, G. Gomišček, V. Arrigler, and H. Hägerstrand. 2002. Microtubes and nanotubes of phospholipid bilayer vesicles. J. Phys. Math. Gen. 35:1533–1549.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

