Substrate modulus directs neural stem cell behavior
- PMID: 18658232
- PMCID: PMC2567955
- DOI: 10.1529/biophysj.108.132217
Substrate modulus directs neural stem cell behavior
Abstract
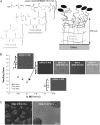
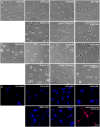
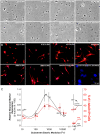
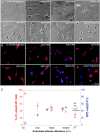
Although biochemical signals that modulate stem cell self-renewal and differentiation were extensively studied, only recently were the mechanical properties of a stem cell's microenvironment shown to regulate its behavior. It would be desirable to have independent control over biochemical and mechanical cues, to analyze their relative and combined effects on stem-cell function. We developed a synthetic, interfacial hydrogel culture system, termed variable moduli interpenetrating polymer networks (vmIPNs), to assess the effects of soluble signals, adhesion ligand presentation, and material moduli from 10-10,000 Pa on adult neural stem-cell (aNSC) behavior. The aNSCs proliferated when cultured in serum-free growth media on peptide-modified vmIPNs with moduli of >/=100 Pa. In serum-free neuronal differentiation media, a peak level of the neuronal marker, beta-tubulin III, was observed on vmIPNs of 500 Pa, near the physiological stiffness of brain tissue. Furthermore, under mixed differentiation conditions with serum, softer gels ( approximately 100-500 Pa) greatly favored neurons, whereas harder gels ( approximately 1,000-10,000 Pa) promoted glial cultures. In contrast, cell spreading, self-renewal, and differentiation were inhibited on substrata with moduli of approximately 10 Pa. This work demonstrates that the mechanical and biochemical properties of an aNSC microenvironment can be tuned to regulate the self-renewal and differentiation of aNSCs.
Figures


References
-
- Hay, E. D. 1982. Interaction of embryonic surface and cytoskeleton with extracellular matrix. Am. J. Anat. 165:1–12. - PubMed
-
- Nakagawa, S., P. Pawelek, and F. Grinnell. 1989. Extracellular matrix organization modulates fibroblast growth and growth factor responsiveness. Exp. Cell Res. 182:572–582. - PubMed
-
- Halliday, N. L., and J. J. Tomasek. 1995. Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Exp. Cell Res. 217:109–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

