Increased FOG-2 in failing myocardium disrupts thyroid hormone-dependent SERCA2 gene transcription
- PMID: 18658259
- PMCID: PMC2650841
- DOI: 10.1161/CIRCRESAHA.108.181487
Increased FOG-2 in failing myocardium disrupts thyroid hormone-dependent SERCA2 gene transcription
Abstract
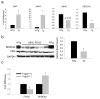
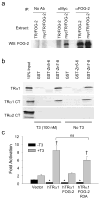
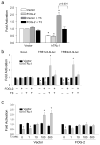
Reduced expression of sarcoplasmic reticulum calcium ATPase (SERCA)2 and other genes in the adult cardiac gene program has raised consideration of an impaired responsiveness to thyroid hormone (T3) that develops in the advanced failing heart. Here, we show that human and murine cardiomyopathy hearts have increased expression of friend of GATA (FOG)-2, a cardiac nuclear hormone receptor corepressor protein. Cardiac-specific overexpression of FOG-2 in transgenic mice led to depressed cardiac function, activation of the fetal gene program, congestive heart failure, and early death. SERCA2 transcript and protein levels were reduced in FOG-2 transgenic hearts, and FOG-2 overexpression impaired T3-mediated SERCA2 expression in cultured cardiomyocytes. FOG-2 physically interacts with thyroid hormone receptor-alpha1 and abrogated even high levels of T3-mediated SERCA2 promoter activity. These results demonstrate that SERCA2 is an important target of FOG-2 and that increased FOG-2 expression may contribute to a decline in cardiac function in end-stage heart failure by impaired T3 signaling.
Figures



Similar articles
-
Restoration of sarcoplasmic reticulum protein level by thyroid hormone contributes to partial improvement of myocardial function, but not to glucose metabolism in an early failing heart.Eur J Cardiothorac Surg. 2003 Oct;24(4):493-501. doi: 10.1016/s1010-7940(03)00410-x. Eur J Cardiothorac Surg. 2003. PMID: 14500065
-
Cardiac hypertrophy and thyroid hormone signaling.Heart Fail Rev. 2010 Mar;15(2):125-32. doi: 10.1007/s10741-008-9125-7. Epub 2009 Jan 6. Heart Fail Rev. 2010. PMID: 19125327 Free PMC article. Review.
-
Thyroid hormone receptor modulates the expression of the rabbit cardiac sarco (endo) plasmic reticulum Ca(2+)-ATPase gene.J Biol Chem. 1994 Jan 14;269(2):1460-7. J Biol Chem. 1994. PMID: 8166809
-
Sp1 and Sp3 transcription factors are required for trans-activation of the human SERCA2 promoter in cardiomyocytes.Cardiovasc Res. 2003 Nov 1;60(2):347-54. doi: 10.1016/s0008-6363(03)00529-7. Cardiovasc Res. 2003. PMID: 14613864
-
Calcium regulatory proteins and their alteration by transgenic approaches.Am J Cardiol. 1999 Jun 17;83(12A):89H-91H. doi: 10.1016/s0002-9149(99)00268-4. Am J Cardiol. 1999. PMID: 10750595 Review.
Cited by
-
Association of serum triiodothyronine with B-type natriuretic peptide and severe left ventricular diastolic dysfunction in heart failure with preserved ejection fraction.Am J Cardiol. 2012 Jul 15;110(2):234-9. doi: 10.1016/j.amjcard.2012.02.068. Epub 2012 Apr 11. Am J Cardiol. 2012. PMID: 22502900 Free PMC article.
-
Myocardial transcription factors in diastolic dysfunction: clues for model systems and disease.Heart Fail Rev. 2016 Nov;21(6):783-794. doi: 10.1007/s10741-016-9569-0. Heart Fail Rev. 2016. PMID: 27306370 Review.
-
A Large-Scale High-Throughput Screen for Modulators of SERCA Activity.Biomolecules. 2022 Nov 30;12(12):1789. doi: 10.3390/biom12121789. Biomolecules. 2022. PMID: 36551215 Free PMC article.
-
Changes in myocardial myosin heavy chain isoform composition with exercise and post-exercise cold-water immersion.J Muscle Res Cell Motil. 2021 Jun;42(2):183-191. doi: 10.1007/s10974-021-09603-z. Epub 2021 Apr 7. J Muscle Res Cell Motil. 2021. PMID: 33826086
-
Micro-RNA expression in hypoplastic left heart syndrome.J Card Fail. 2015 Jan;21(1):83-8. doi: 10.1016/j.cardfail.2014.09.013. Epub 2014 Oct 5. J Card Fail. 2015. PMID: 25291457 Free PMC article.
References
-
- Lowes BD, Minobe W, Abraham WT, Rizeq MN, Bohlmeyer TJ, Quaife RA, Roden RL, Dutcher DL, Robertson AD, Voelkel NF, Badesch DB, Groves BM, Gilbert EM, Bristow MR. Changes in gene expression in the intact human heart. Downregulation of alpha-myosin heavy chain in hypertrophied, failing ventricular myocardium. J Clin Invest. 1997;100:2315–24. - PMC - PubMed
-
- Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–9. - PubMed
-
- van Tuyl M, Blommaart PE, de Boer PA, Wert SE, Ruijter JM, Islam S, Schnitzer J, Ellison AR, Tibboel D, Moorman AF, Lamers WH. Prenatal exposure to thyroid hormone is necessary for normal postnatal development of murine heart and lungs. Dev Biol. 2004;272:104–17. - PubMed
-
- Arai M, Otsu K, MacLennan DH, Alpert NR, Periasamy M. Effect of thyroid hormone on the expression of mRNA encoding sarcoplasmic reticulum proteins. Circ Res. 1991;69:266–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

