Synaptic pathology in retinoschisis knockout (Rs1-/y) mouse retina and modification by rAAV-Rs1 gene delivery
- PMID: 18660429
- PMCID: PMC2556260
- DOI: 10.1167/iovs.07-1071
Synaptic pathology in retinoschisis knockout (Rs1-/y) mouse retina and modification by rAAV-Rs1 gene delivery
Abstract
Purpose: At an early age, the retinoschisin knockout (Rs1-KO) mouse retina has progressive photoreceptor degeneration with severe disruption of the outer plexiform layer (OPL) that decreases at older ages. The electroretinogram (ERG) undergoes parallel changes. The b-wave amplitude from bipolar cells is reduced disproportionately to the photoreceptor a-wave at young but not at older ages. The protein expression and morphology of the OPL in Rs1-KO mice was investigated at different ages, to explore the role of the synaptic layer in these ERG changes.
Methods: Retinas of wild-type (Wt) and Rs1-KO mice from postnatal day (P)7 to 12 months were evaluated by light and electron microscopy (EM) and biochemistry. PSD95 (postsynaptic density protein), mGluR6 (metabotropic glutamate receptor subtype 6), retinoschisin (Rs1), the Müller cell proteins glial fibrillary acidic protein (GFAP) and glutamine synthetase (GS), the bipolar cell marker protein kinase C alpha (PKCalpha), and the horizontal cell marker calbindin were localized by immunofluorescence and immuno-EM. Levels of PSD95 and mGluR6 were determined by quantitative Western blot. Rs1-KO mice treated by intravitreous injection of rAAV(2/2)-CMV-Rs1 in one eye at P14 were evaluated at 8 months by full-field scotopic ERG responses and retinal immunohistochemistry.
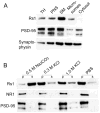
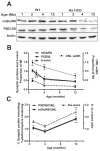
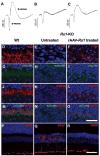
Results: Rs1 was associated with the outer surface of synaptic membranes in wild-type (Wt) retinas. PSD95 and mGluR6 were juxtaposed in the OPL of the Rs1-KO retinas by P14, implying that synaptic structures are formed. Light microscopic retinal morphology was similar in Wt and Rs1-KO at P14, but by P21, the OPL was disrupted in Rs1-KO, and some PSD95 and mGluR6 was mislocalized in the outer nuclear layer (ONL). GFAP expression spanned all retinal layers. EM showed synaptic structures adjacent to photoreceptor nuclei. PSD95 and mGluR6 levels were normal at 1 month on Western blot but declined to 59% (P < 0.001) and 55% (P < 0.05) of Wt, respectively, by 4 months. Levels thereafter showed no further reduction out to 12 months. Eyes injected with AAV-Rs1 were studied at 8 months by immunohistochemistry and had higher expression of PSD95 and mGluR6 and less GFAP expression compared with fellow untreated eyes.
Conclusions: In the Rs1-KO mouse, retinal layer formation and synaptic protein expression in the OPL is normal up to P14, implying normal development of synaptic connections. Aberrant localization of synaptic proteins by P21 indicates that displacement of developing and/or mature synapses contributes to the b-wave reduction at young ages, when photoreceptor numbers and synaptic protein levels are normal. The subsequent decline in PSD95 and mGluR6 between 1 and 12 months in Rs1-KO retina mirrors the course of b-wave change and provides evidence of causal relationship between the ERG and OPL changes. These findings and the improved structural integrity of the OPL and b-wave amplitude after Rs1 gene transfer therapy provide a cellular and molecular basis for interpreting the changes in retinal signaling in this model.
Figures





References
-
- Sauer CG, Gehrig A, Warneke-Wittstock R, et al. Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nat Genet. 1997;17:164–170. - PubMed
-
- Takada Y, Fariss RN, Tanikawa A, et al. A retinal neuronal developmental wave of retinoschisin expression begins in ganglion cells during layer formation. Invest Ophthalmol Vis Sci. 2004;45:3302–3312. - PubMed
-
- Takada Y, Fariss RN, Müller M, Bush RA, Rushing EJ, Sieving PA. Retinoschisin expression and localization in rodent and human pineal and consequences of mouse RS1 gene knockout. Mol Vis. 2006;12:1108–1116. - PubMed
-
- Vogel W. Discoidin domain receptors: structural relations and functional implications. FASEB J. 1999;13(suppl):S77–S82. - PubMed
-
- Wu WW, Wong JP, Kast J, Molday RS. RS1, a discoidin domain-containing retinal cell adhesion protein associated with X-linked retinoschisis, exists as a novel disulfide-linked octamer. J Biol Chem. 2005;280:10721–10730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

