The population genetics of using homing endonuclease genes in vector and pest management
- PMID: 18660532
- PMCID: PMC2516076
- DOI: 10.1534/genetics.108.089037
The population genetics of using homing endonuclease genes in vector and pest management
Abstract
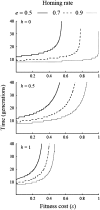
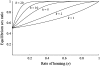
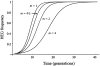
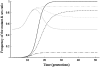
Homing endonuclease genes (HEGs) encode proteins that in the heterozygous state cause double-strand breaks in the homologous chromosome at the precise position opposite the HEG. If the double-strand break is repaired using the homologous chromosome, the HEG becomes homozygous, and this represents a powerful genetic drive mechanism that might be used as a tool in managing vector or pest populations. HEGs may be used to decrease population fitness to drive down population densities (possibly causing local extinction) or, in disease vectors, to knock out a gene required for pathogen transmission. The relative advantages of HEGs that target viability or fecundity, that are active in one sex or both, and whose target is expressed before or after homing are explored. The conditions under which escape mutants arise are also analyzed. A different strategy is to place HEGs on the Y chromosome that cause one or more breaks on the X chromosome and so disrupt sex ratio. This strategy can cause severe sex-ratio biases with efficiencies that depend on the details of sperm competition and zygote mortality. This strategy is probably less susceptible to escape mutants, especially when multiple X shredders are used.
Figures






References
-
- Alphey, L., C. B. Beard, P. Billingsley, M. Coetzee, A. Crisanti et al., 2002. Malaria control with genetically manipulated insect vectors. Science 298 119–121. - PubMed
-
- Arnould, S., C. Perez, J. P. Cabaniols, J. Smith, A. Gouble et al., 2007. Engineered I–Crel derivatives cleaving sequences from the human XPC gene can induce highly efficient gene correction in mammalian cells. J. Mol. Biol. 371 49–65. - PubMed
-
- Ashburner, M., K. G. Golic and R. S. Hawley, 2005. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

