Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress
- PMID: 18660816
- PMCID: PMC4095974
- DOI: 10.1038/nm.1853
Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress
Abstract
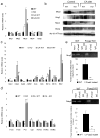
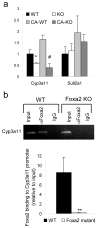
Production of bile by the liver is crucial for the absorption of lipophilic nutrients. Dysregulation of bile acid homeostasis can lead to cholestatic liver disease and endoplasmic reticulum (ER) stress. We show by global location analysis ('ChIP-on-chip') and cell type-specific gene ablation that the winged helix transcription factor Foxa2 is required for normal bile acid homeostasis. As suggested by the location analysis, deletion of Foxa2 in hepatocytes in mice using the Cre-lox system leads to decreased transcription of genes encoding bile acid transporters on both the basolateral and canalicular membranes, resulting in intrahepatic cholestasis. Foxa2-deficient mice are strikingly sensitive to a diet containing cholic acid, which results in toxic accumulation of hepatic bile salts, ER stress and liver injury. In addition, we show that expression of FOXA2 is markedly decreased in liver samples from individuals with different cholestatic syndromes, suggesting that reduced FOXA2 abundance could exacerbate the injury.
Conflict of interest statement
Figures




References
-
- Kaestner KH. The hepatocyte nuclear factor 3 (HNF3 or FOXA) family in metabolism. Trends Endocrinol Metab. 2000;11:281–5. - PubMed
-
- Ang SL, et al. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development. 1993;119:1301–15. - PubMed
-
- Monaghan AP, Kaestner KH, Grau E, Schutz G. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development. 1993;119:567–78. - PubMed
-
- Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

