Uptake kinetics and biodistribution of 14C-D-luciferin--a radiolabeled substrate for the firefly luciferase catalyzed bioluminescence reaction: impact on bioluminescence based reporter gene imaging
- PMID: 18661130
- PMCID: PMC4157642
- DOI: 10.1007/s00259-008-0870-6
Uptake kinetics and biodistribution of 14C-D-luciferin--a radiolabeled substrate for the firefly luciferase catalyzed bioluminescence reaction: impact on bioluminescence based reporter gene imaging
Abstract
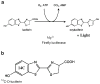
Purpose: Firefly luciferase catalyzes the oxidative decarboxylation of D: -luciferin to oxyluciferin in the presence of cofactors, producing bioluminescence. This reaction is used in optical bioluminescence-based molecular imaging approaches to detect the expression of the firefly luciferase reporter gene. Biokinetics and distribution of the substrate most likely have a significant impact on levels of light signal and therefore need to be investigated.
Methods: Benzene ring (14)C(U)-labeled D-luciferin was utilized. Cell uptake and efflux assays, murine biodistribution, autoradiography and CCD-camera based optical bioluminescence imaging were carried out to examine the in vitro and in vivo characteristics of the tracer in cell culture and in living mice respectively.
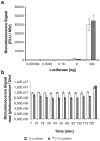
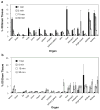
Results: Radiolabeled and unlabeled D-luciferin revealed comparable levels of light emission when incubated with equivalent amounts of the firefly luciferase enzyme. Cell uptake assays in pCMV-luciferase-transfected cells showed slow trapping of the tracer and relatively low uptake values (up to 22.9-fold higher in firefly luciferase gene-transfected vs. nontransfected cells, p = 0.0002). Biodistribution studies in living mice after tail-vein injection of (14)C-D-luciferin demonstrated inhomogeneous tracer distribution with early predominant high radioactivity levels in kidneys (10.6% injected dose [ID]/g) and liver (11.9% ID/g), followed at later time points by the bladder (up to 81.3% ID/g) and small intestine (6.5% ID/g), reflecting the elimination routes of the tracer. Kinetics and uptake levels profoundly differed when using alternate injection routes (intravenous versus intraperitoneal). No clear trapping of (14)C-D-luciferin in firefly luciferase-expressing tissues could be observed in vivo.
Conclusions: The data obtained with (14)C-D-luciferin provide insights into the dynamics of D: -luciferin cell uptake, intracellular accumulation, and efflux. Results of the biodistribution and autoradiographic studies should be useful for optimizing and adapting optical imaging protocols to specific experimental settings when utilizing the firefly luciferase and D: -luciferin system.
Conflict of interest statement
Figures





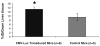
References
-
- Contag CH, Ross BD. It’s not just about anatomy: in vivo bioluminescence imaging as an eyepiece into biology. J Magn Reson Imaging. 2002;16:378–87. - PubMed
-
- Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17(5):545–80. - PubMed
-
- Iyer M, Sato M, Johnson M, Gambhir SS, Wu L. Applications of molecular imaging in cancer gene therapy. Curr Gene Ther. 2005;5 (6):607–18. - PubMed
-
- Tannous BA, Kim DE, Fernandez JL, Weissleder R, Breakefield XO. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther. 2005;11(3):435–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

